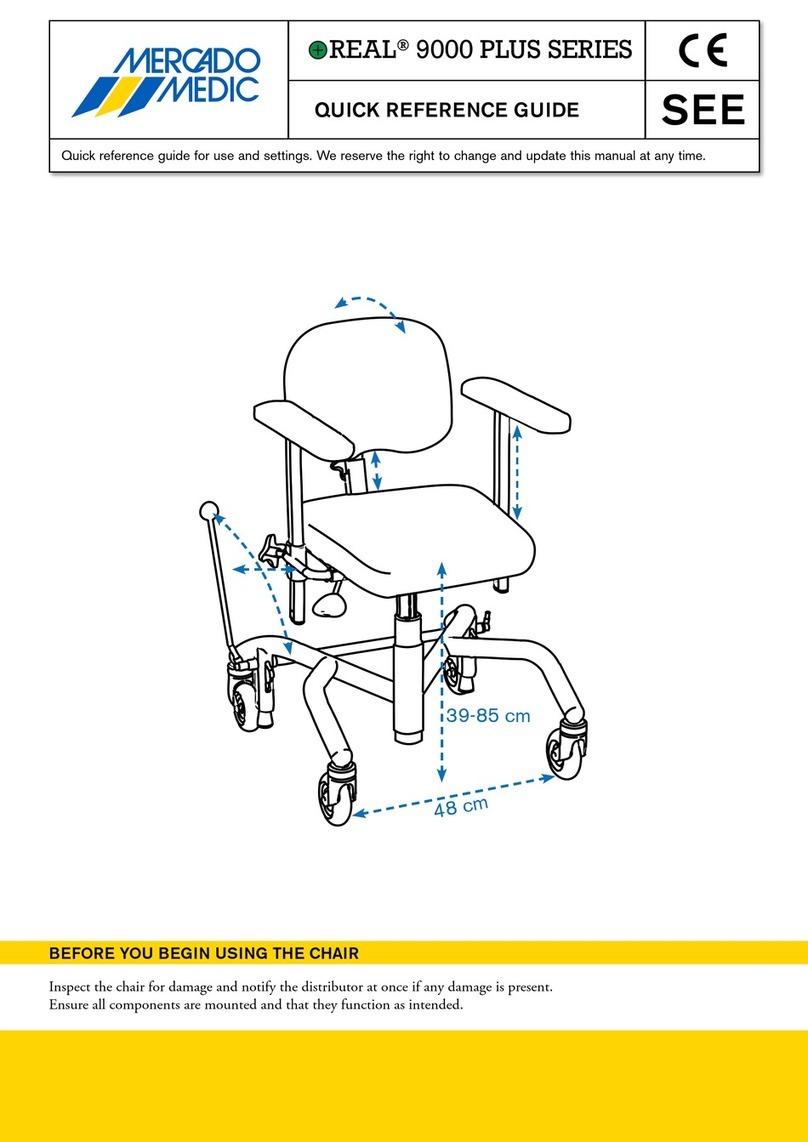
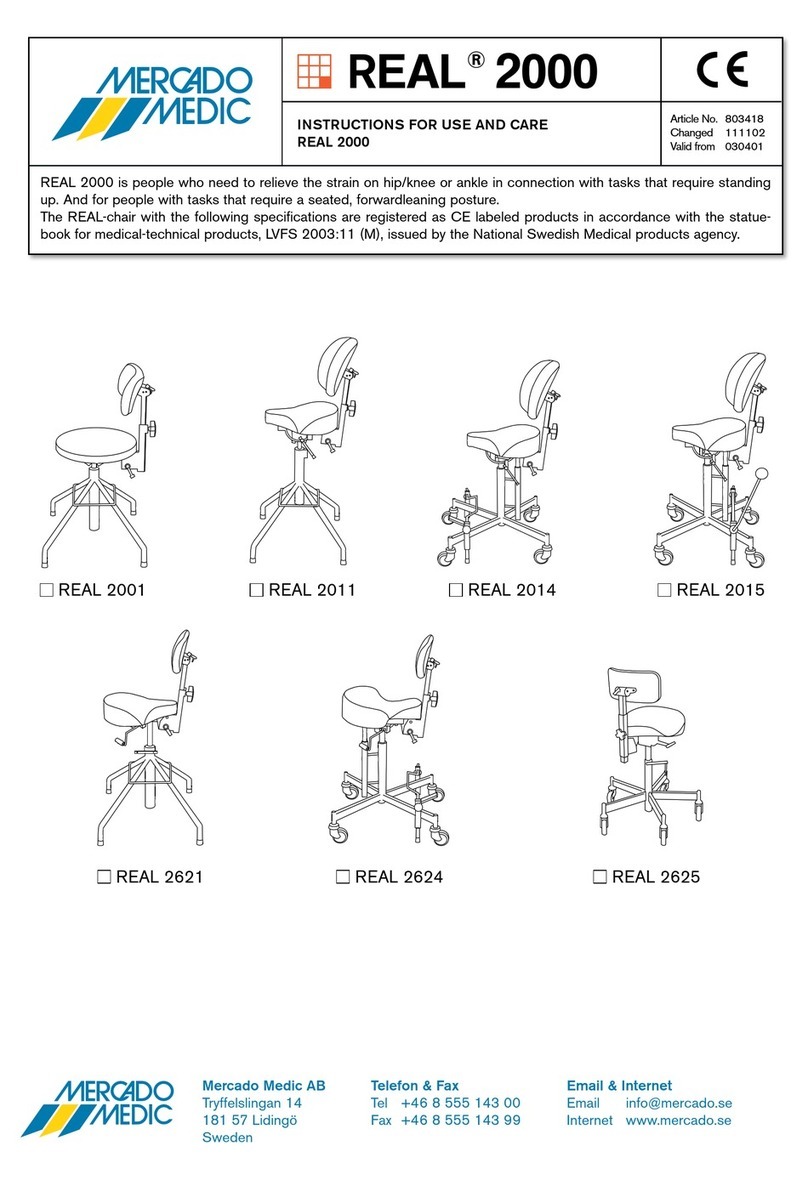
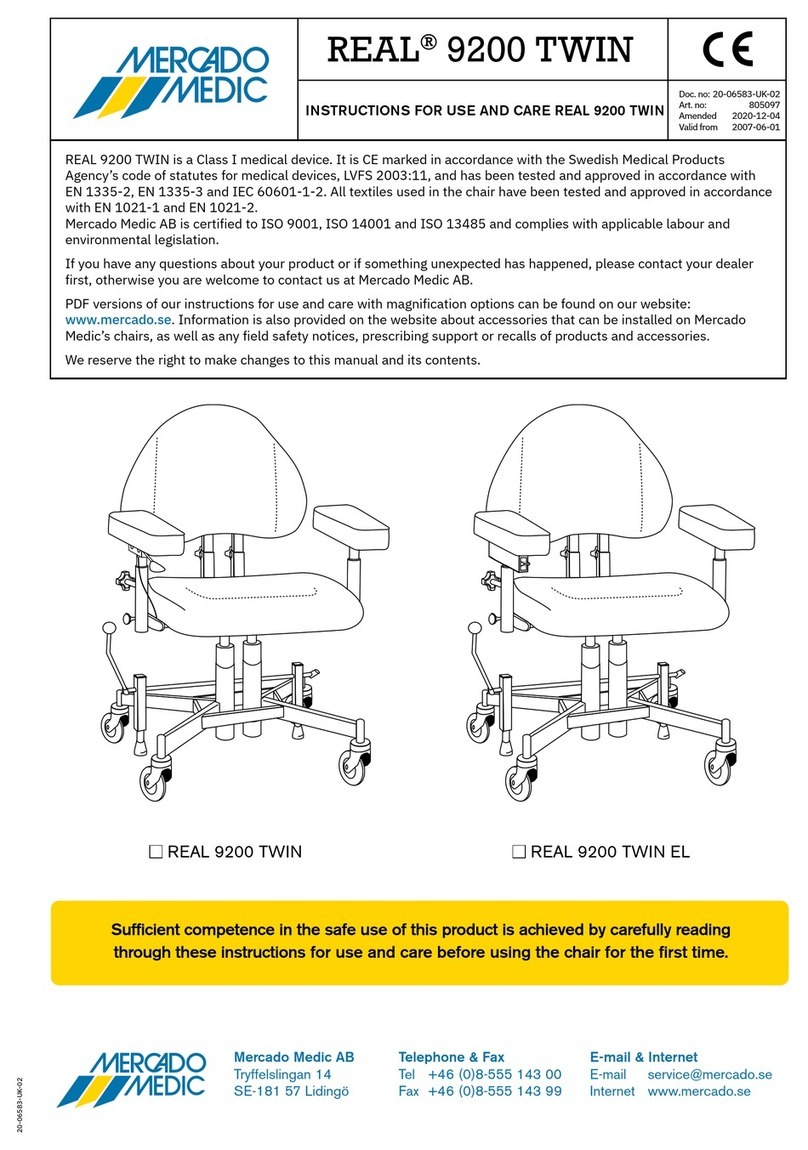
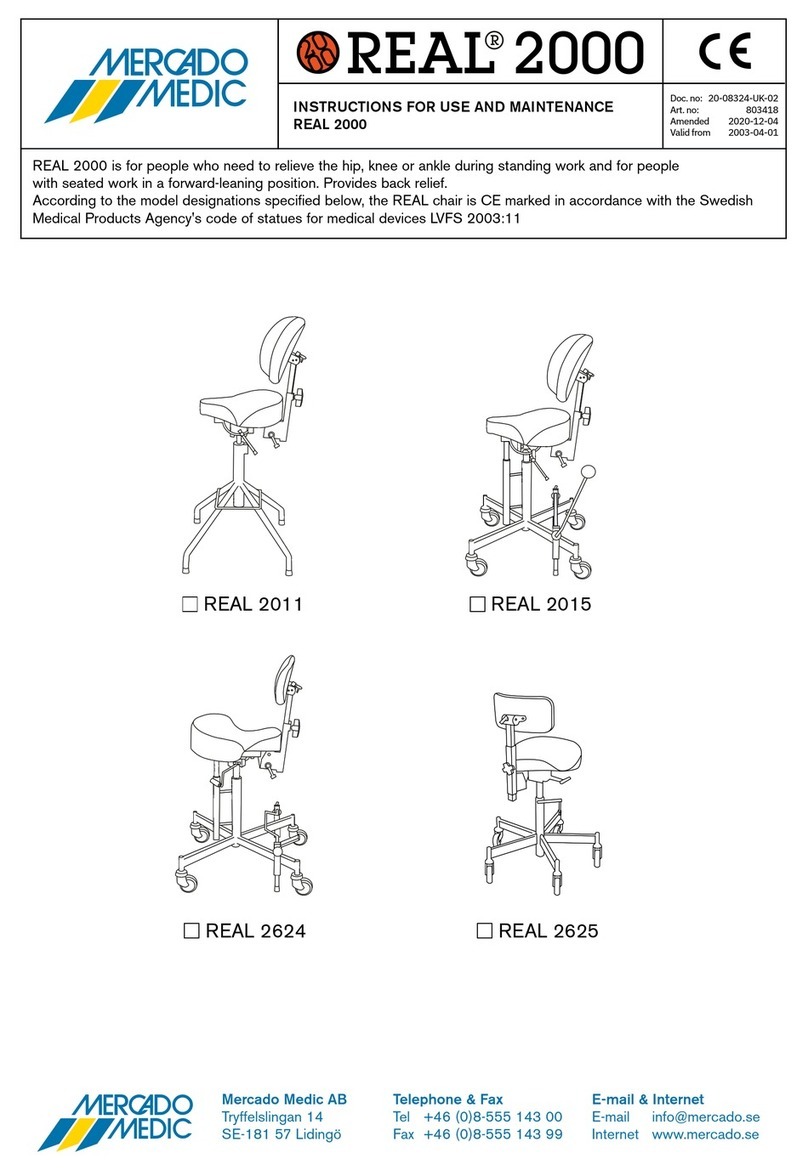
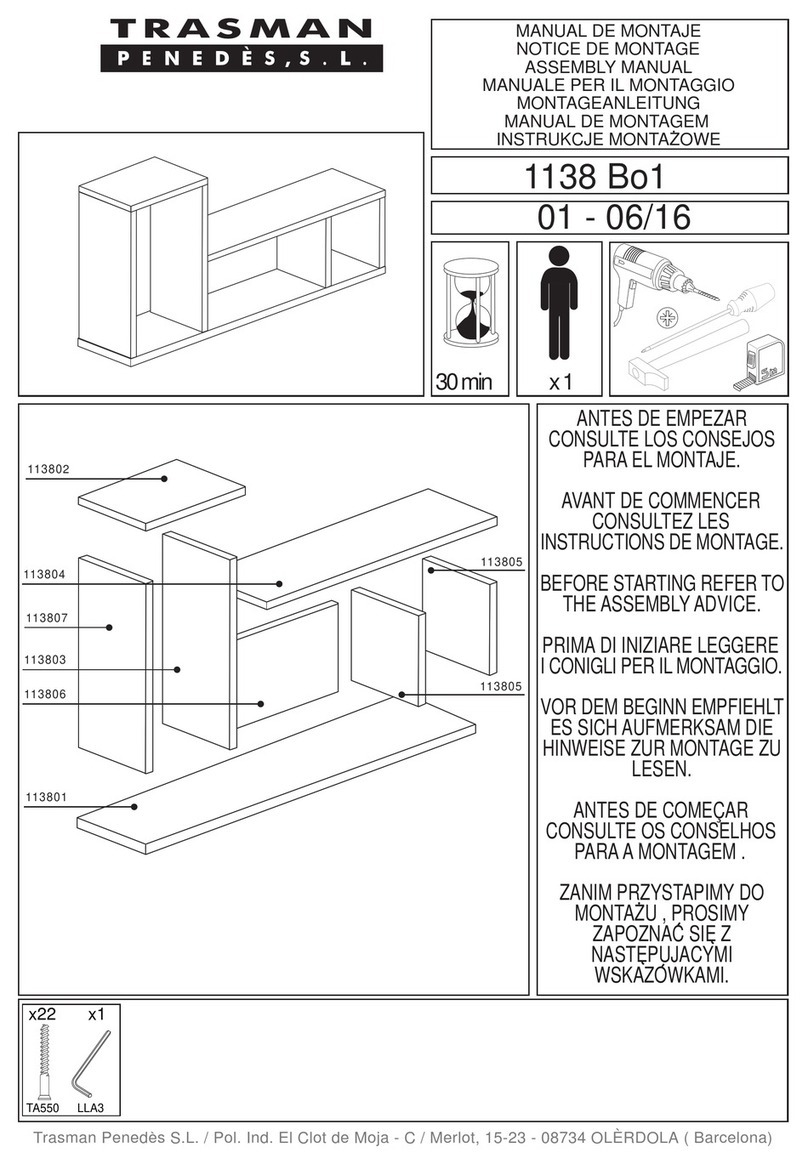
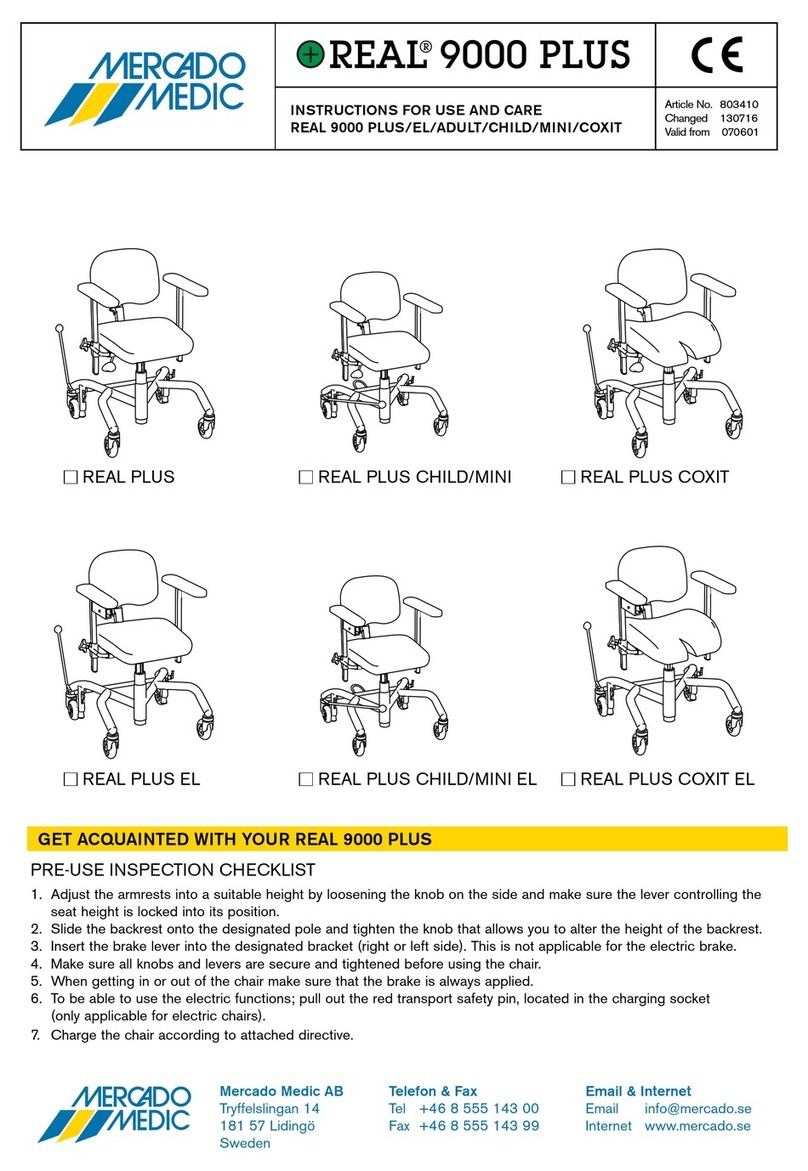
3
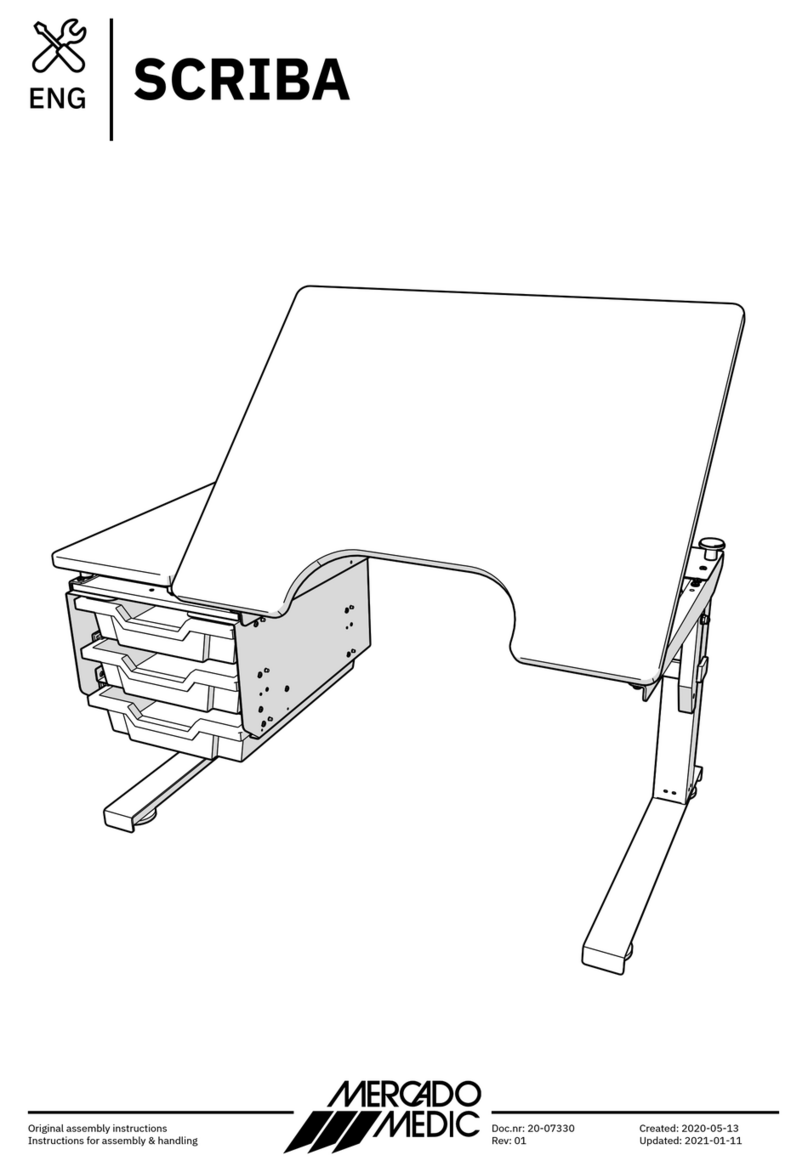
SCRIBA
19-05994-02
1. Important information
1.1. Regarding use and environment
The table is intended for indoor use only and must not be used outdoors. The table must not be exposed to
extreme cold or heat, prolonged sunlight or other radiation. The table must not be exposed to water, other fluids
or chemicals. Warning: Metal surfaces can become very hot if they have been exposed to sunlight. The table must
not be equipped with accessories or components other than the ones approved by Mercado Medic. To maintain
the CE marking, modifications or changes must not be carried out without Mercado Medic’s approval.
• Sufficient competence to use this product safely
is obtained by carefully reading these instructions
for use and care before using the table.
• Repairs and other technical measures
may only be carried out by personnel
authorised by Mercado Medic.
• Warranty period is two (2) years unless
otherwise agreed. For warranty cases,
please contact Mercado Medic.
• Expected service life is ten (10) years.
• Loose parts must be secured
prior to transportation.
• Depending on configuration, the
table weighs 25-45 kg.
• The table does not generate noise
levels above 70 db (A)
• The table is intended for use between +5 and
+40°C and 15% to 90% non-condensing humidity.
1.2. Warnings
Some configurations have split table tops. Risk of crushing may occur when the tiltable part is lowered.
1.3. Declaration of Conformity
Mercado Medic hereby declare under our sole responsibility as a manufacturer that the product identified below,
Product name: SCRIBA
General Description: A height-adjustable desk with configuration possibilities providing
drawers, different tabletop designs and a tilt mechanism for the tabletop. The product is
designed, however not exclusively, for use by children in school environments.
conform to the provisions of the following EC directive and regulation:
Directive 2006/42/EC Machinery
The following relevant harmonised standards were applied in the design and development of the product:
• SS-EN ISO 13857:2008
• SS-EN ISO 14971:2012*
• EN 349 + A1:2008
• EN 614-1:2006 + A1:2009
* The standard for risk assessment is harmonised with Directive 93/42/EC Medical Devices. Although the SCRIBA is not a medical device, Mercado Medic AB is a
manufacturer of medical devices and consider the standard to be appropriate and applicable.