MicroAire 7500-700 User manual

7500-700 Battery Charger
Instructions for Use

IM-7500-700 Rev K 2021-05 2 of 12
Technical Description ........................................................................ 2
Intended Use and Introduction............................................................... 2
General Warnings.......................................................................... 3-4
Symbol Definitions......................................................................... 4-5
Compatible Instruments ..................................................................... 6
Setup and Operation......................................................................... 6
Cleaning/Decontamination .................................................................. 7
Shipping and Environmental Parameters ..................................................... 7
Troubleshooting ............................................................................. 7
Disposal/Recycle............................................................................. 8
Services and Repair .......................................................................... 8
Warranty .................................................................................... 8
Electromagnetic Compatibility ............................................................9-10
Table of Contents
REF 7500-700 Battery Charger Instruction Manual
Technical Description
REF 7500-700
Ratings:
• Class 1 Equipment
• Input Rating: 100-240 V~, 50-60 Hz, 0.30 Kw
Output Rating: 24 V DC, 150 W
Intended Use
The MicroAire REF 7500-700 Battery Charger is intended to charge all MicroAire 14.4 volt NiMH battery packs.
Introduction
This manual was written to help describe the procedures required to keep the MicroAire Battery Charger system
operating properly. Please read this manual and follow its instructions carefully. The words WARNING, CAUTION and
NOTE carry special meanings and should be carefully reviewed.

IM-7500-700 Rev K 2021-05
3 of 12
WARNING: Used to indicate that the safety of the patient and hospital personnel could be involved.
CAUTION: Used to indicate special procedures or precautions that must be followed to avoid damaging the
system and instruments.
NOTE: Used to indicate the easiest means of carrying out techniques.
General Warnings:
WARNING: Risk of re. Replace fuse with T5A/250V.
WARNING: Risk of re. Replace battery pack only with a MicroAire REF 6640-710 Battery Pack, REF 7505-710 or
REF 7500-620 Aseptic Battery Pack. Always use the REF 7500-625 Charger Adapter for the Aseptic Pack.
WARNING: Explosion hazard. Not suitable for use in the presence of ammable anesthetics or oxygen.
WARNING: Electric shock. Do not remove cover. Refer servicing to authorized MicroAire personnel only.
WARNING: Main disconnect must be achieved by removing cord from wall outlet.
WARNING: Use of the REF 7500-700 Battery Charger adjacent to or stacked with other equipment should
be avoided because it could result in improper operation. If such use is necessary, the REF 7500-700
Battery Charger and the adjacent equipment should be observed to verify that they are operating
normally.
WARNING: Use only with a medically approved Type SJT or equivalent three-wire grounded power cord rated for
125V or 250V AC, 10A, constructed with 18/3 AWG wires and an IEC 320 appliance connector and a
Hospital Grade outlet plug. The cord length shall not exceed 10ft (3.05m).
WARNING: Grounding reliability can only be achieved when the equipment is connected to an equipment
receptacle marked“Hospital Only”or “Hospital Grade”.
WARNING: No modication of this equipment is allowed.
WARNING: Prior to use system components should be inspected and operated to detect any damage or
malfunction. Do not use if damage is apparent.
WARNING: Medical electrical equipment may be aected by electromagnetic interference. It should be installed
and used in accordance with the electromagnetic compatibility information provided herein.
WARNING: Portable RF communications equipment should be used no closer than 30 cm (12 inches) away from
any part of the REF 7500-700 Battery Charger, the batteries being charged, or its power cable. Other
wise degradation of the performance of battery charger could result.
WARNING: Use with Battery Packs other than the REF 6640-710, REF 7505-710, or REF 7500-620 used with a
REF 7500-625 Charger Adapter could result in increased electromagnetic emissions or decreased
electromagnetic immunity, resulting in improper operation of the REF 7500-700 Battery Charger.
WARNING: The following items should be periodically inspected for signs of damage and repaired or replaced as
needed to ensure continued safety with regard to electromagnetic disturbances over the life of the
REF 7500-700 Battery Charger:
• Verify that all enclosure panels are securely fastened in place.
• Test that the Protective Earth Connection meets ES60601-1 Requirements.
WARNING: The REF 7500-700 Battery Charger is suitable for use in hospitals and surgery centers. The Battery
Charger should not be used near High Frequency Surgical equipment or Magnetic Resonance
Imaging equipment.
IM-7500-700 Rev K 2021-05 4 of 12
CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician (or properly
licensed practitioner).
CAUTION: Do not autoclave.
CAUTION: Do not immerse in liquids to cool.
CAUTION: Do not ash sterilize.
CAUTION: Do not use Ethylene Oxide Sterilization.
CAUTION: Do not use a washer sterilizer.
CAUTION: Do not process in equipment using peracetic acid.
NOTE: The emissions characteristics of this product make it suitable for use in industrial areas and hospitals
(CISPR 11 Class A). If it is used in a residential environment (for which CISPR 11 Class Bis normally
required) this equipment might not offer adequate protection to radio frequency communication
services. The user might be required to take mitigation measures, such as relocating or re-orienting
the equipment.
NOTE: The Battery Charger is designed for use only with MicroAire Batteries REF 6640-710, REF 7505-710
and REF 7500-620 Aseptic Battery using a REF 7500-625 charger adapter.
NOTE: All personnel should become familiar with the power equipment before it is set-up for use in any
procedure. Personnel in-serviced should include, but not limited to, central processing personnel,
members of the surgical team, and the bioengineering department.
NOTE: DO NOT return equipment without an RMA number. Doing so could cause delays in service, and/or
problems tracking your equipment.outside the U.S. unless specied otherwise.
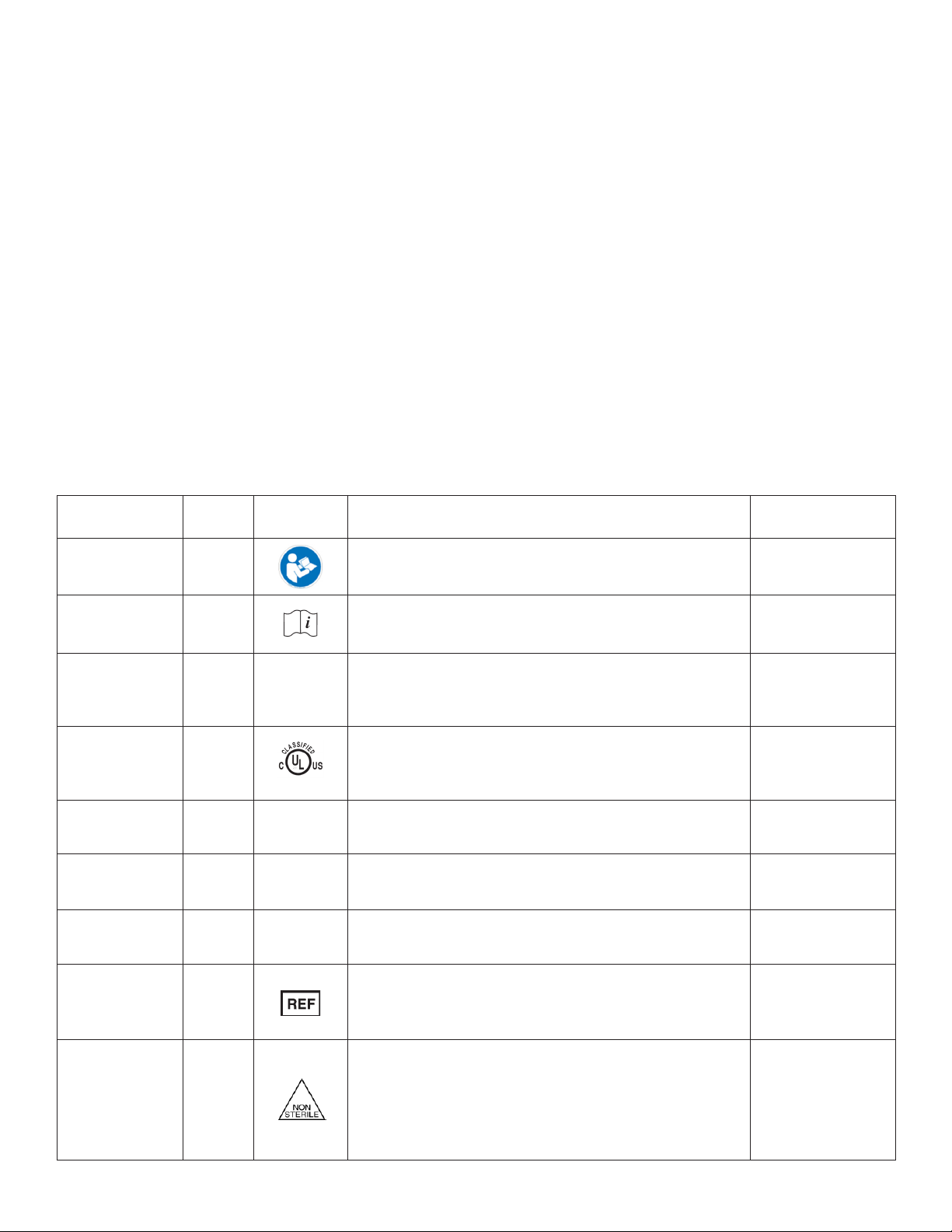
Name Ref#
(ISO 7000)2Symbol Description Use Standard
Refer to Instruction
Manual / Booklet
ISO-7010
M002
• Indicates a MANDATORY action for the user to consult the
Instructions For Use (IFU).
• Symbol must be blue, as shown.
IEC 60601-1:20051
Consult
Instructions For
Use (IFU)
1641
Indicates the need for the user to consult the Instructions For Use
(IFU). Not required in conjunction with the Caution
symbol, if applicable.
ISO 15223-1:20121
Caution
0434A /
0434B CIndicates the need for the user to consult the Instructions For Use
(IFU) for important cautionary information such as warnings and
precautions that cannot, for a variety of reasons, be presented on
the device itself.
ISO 15223-1:20121
UL symbol N/A
E494242
MEDICAL-GENERAL MEDICAL EQUIPMENT AS TO ELECTRIC SHOCK,
FIRE, AND MECHANICAL HAZARDS ONLY. IN ACCORDANCE WITH
ANSI/AAMI ES 60601-1 (2005) + A1 (2012) + CAN/CSA C22.2 No.
60601-1 (2014) | Control Number: E494242
UL
Charge Complete
(Battery) N/A Indicates that charging of battery has completed. N/A
Charge Failed
(Battery) N/A Indicates that charging of battery has failed. N/A
Charging (Battery) N/A Indicates that charging of battery is in progress. N/A
REF (Catalog #) 2493
• Indicates the manufacturer’s catalog number so that the medical
device can be identied.
• Per EN980:2008, the REF symbol may be used without
surrounding box.
ISO 15223-1:20121
Non-Sterile 2609
• Indicates a medical device that has not been subjected to a
sterilization process. This symbol should only be used to
distinguish between identical or similar medical devices sold in
both sterile and non-sterile conditions.
• Also indicates a medical device that is provided non-sterile but
must be sterilized prior to use.
IEC 60601-1:20051
IM-7500-700 Rev K 2021-05
5 of 12
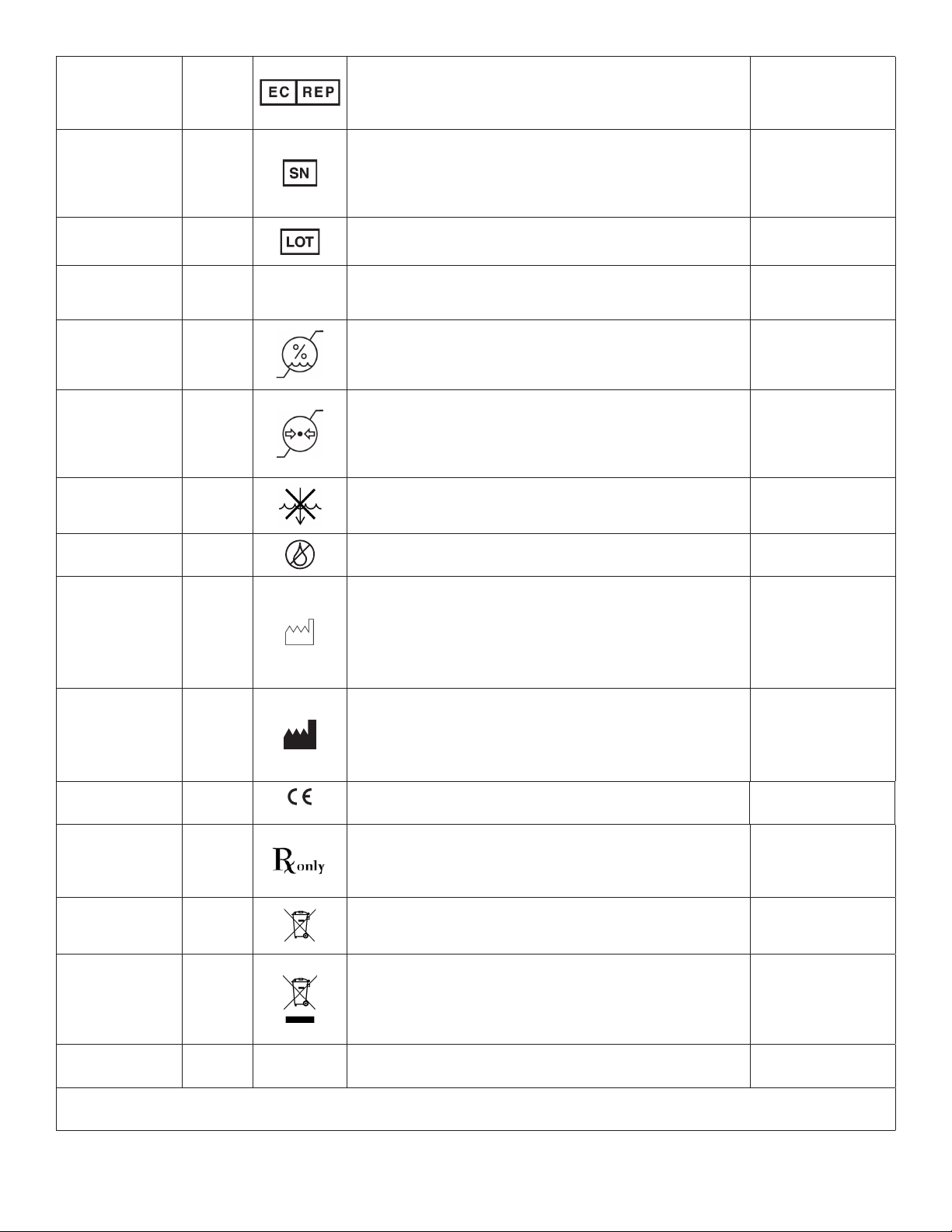
Authorized
Representative
in the European
Community
N/A
Indicates the authorized representative in the European
Community. This symbol shall be accompanied by the name and
address of the authorized representative, adjacent to the symbol.
ISO 15223-1:20121
Serial # 2498
• Indicates the manufacturer’s serial number so that a specic
medical device can be identied.
• Per EN980:2008, the SN symbol may be used without
surrounding box.
ISO 15223-1:20121
Lot / Batch Code 2492 Indicates the manufacturer’s batch code so that the batch or lot
can be identied. ISO 15223-1:20121
Standby 5266 Indicates stand-by or preparatory state for a part of a piece of
equipment. IEC 60878:2015
Humidity Limita-
tion 2620
Indicates the range of humidity to which the medical device can be
safely exposed. The humidity limitations shall be indicated
adjacent to the upper and lower horizontal lines.
ISO 15223-1:20121
Atmospheric
Pressure
Limitation
2621
Indicates the range of atmospheric pressure to which the medical
device can be safely exposed. The atmospheric pressure
limitations shall be indicated adjacent to the upper and lower
horizontal lines.
ISO 15223-1:20121
Do Not Immerse in
any Liquid 5995 Indicates a medical device that is not to be immersed in any liquid. IEC 60335-2-15
Do Not Lubricate N/A Indicates a medical device that is not to be lubricated. N/A
Date of
Manufacture 2497
• Indicates the date when the medical device was
manufactured. The date is expressed as YYYY-MM (e.g. 2015-11)
or YYYY-MM-DD (e.g. 2015-11-29).
• If the symbol is lled (see Manufacturer symbol), both
the date of manufacture and the name/address of the
manufacturer may be combined in one symbol.
ISO 15223-1:20121
Manufacturer 3082
• Indicates the medical device manufacturer. This symbol shall be
accompanied by the name and address of the manufacturer. The
date of manufacture may be combined with this symbol.
• When using MicroAire as the manufacturer, use the MicroAire
LLC symbol.
ISO 15223-1:20121
CE Mark with NB N/A
2797
Indicates the European Conformity Mark with Notied Body
Number. 2797 is the BSI-NL-registered Notied Body.
Council Directive
93/42/EEC
Prescription N/A Caution: Federal Law (U.S.A.) restricts this device to sale by or on
the order of a physician (or properly licensed practitioner).
FDA Title 21,
Chapter 1,
Subchapter H,
Part 801.15(F)
Dispose of per
WEEE Directive
2012/19/EU
N/A
Indicates a medical device that is not to be disposed of as
unsorted municipal waste. Medical device is to be disposed of per
WEEE Directive 2012/19/EU.
Council Directive
2012/19/EU
Dispose of per
WEEE Directive
2012/19/EU
N/A
Indicates a medical device that is not to be disposed of as
unsorted municipal waste. Medical device is to be disposed of per
WEEE Directive 2012/19/EU. This symbol is used in place of the
above symbol if the product entered the market after 13 August,
2005.
Council Directive
2012/19/EU
(Symbol: European
Standard EN 50419)
Packaging is
Recyclable 1135 Indicates that the marked item or its material is part of a recovery
or recycling process. IEC 60878:2015
1 ISO 15223-1:2012 – “Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements”
2 ISO 7000/IEC 60417 – “Graphical symbols for use on equipment – Registered symbols”
Other manuals for 7500-700
1
Table of contents
Other MicroAire Batteries Charger manuals