Neurotech MediStim XP User manual

MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 3

CONTENTS
2
TABLE OF CONTENTS
1.0 Safety Information ___________________________________________________
2.0 Contra-Indications ___________________________________________________
2.1 Precautions & Warnings ________________________________________________
2.2 Warranty _____________________________________________________________
3.0 Description Of Unit And Controls ______________________________________
4.0 Description Of Unit And Display _______________________________________
5.0 Step By Step Treatment Guide ________________________________________
6.0 System Maintenance _________________________________________________
6.1 Accessories __________________________________________________________
7.0 Troubleshooting ______________________________________________________
8.0 Technical Specifications ______________________________________________
9.0 Program Information _________________________________________________
3
4
4
6
7
8
9
12
13
14
15
17
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 4

3
1.0 Safety Information
Validity
The information and technical data contained in this document relates to the MediStim®XP muscle stimulator provided with this
manual. Each MediStim XP unit is attributed a serial number which is located on the back of the unit.
The information and technical data disclosed in this document are proprietary to Bio-Medical Research Ltd. (BMR Ltd) and may
only be used and disseminated for the purposes and to the extent specifically authorised in writing by the company.
Disclaimers
All items of equipment manufactured and sold by BMR Ltd are rigorously checked and tested prior to shipment. However the use of
these units is beyond the area of the company’s control. BMR Ltd only accepts responsibility for the safety, reliability and performance
of the equipment when it is operated in accordance with the instructions herein and within the given specifications. Therefore, the
user must bear full responsibility for any actions arising out of the use or misuse of this equipment. Any modifications, repairs or
servicing must be undertaken by authorised BMR Ltd personnel.
This manual is intended for the guidance of the clinician, who should also decide the location of the electrodes.
The MediStim XP unit is designed by and manufactured for:
Bio-Medical Research Ltd., Parkmore Business Park West, Galway, Ireland
Restrictions
The sale and/ or operation of this equipment is subject to legislation in a number of localities. Compliance with this legislation
rests with the importer, dealer, or user of the equipment as appropriate.
SAFETY INFORMATION
English
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 5

Intended Use:
MediStim XP applies muscle and nerve stimulation using the principles of Neuromuscular Electrical Nerve Stimulation (NMES)
which are defined below. The unit sends short electrical impulses through the surface of the skin via adhesive electrodes.
Neuromuscular Electrical Stimulation (NMES):
NMES may be defined as the application of electrical stimulation of the peripheral nervous system to contract a muscle either
through the direct activation of the motor neurons in the mixed peripheral nerve, or indirectly through reflex recruitment.
Transcutaneous Electrical Nerve Stimulation (TENS)
TENS is a pain therapy based on the application of electrical stimuli to the skin via stimulation of the nerve fibers. There are two
methods: The “pain gate” theory, which blocks the pain signals to the brain, and/or through the increased release of endorphins,
which inhibits the emergence of pain.
Indications:
• Detones muscle spasticity.
• Prevention or retardation of disuse muscle atrophy.
• The symptomatic relief and management of acute pain.
2.0 Contra-Indications
• Patients with electronic implants (e.g., pacemakers and defibrillators) may not use the unit!
2.1 Precautions & Warnings
• If in doubt, always seek medical advice.
• Caution is recommended in patients diagnosed with heart problems or suspected heart problems.
• Medical advice must be obtained before use on persons who are insulin-dependent diabetics or for persons who are under medical
supervision for any cognitive dysfunction.
• Medical opinion must be obtained before persons with any serious illness or injury apply muscle stimulation.
• Safety has not been established for the use of muscle stimulation by pregnant women, persons with cancer or epileptics.
• Do not apply stimulation in the region of recent surgery without medical approval.
•
Avoid applying over recent scars or on broken or inflamed skin, areas of infection,
areas susceptible to acne, thrombosis or other
vascular problems (e.g. broken veins or varicose veins).
• Avoid placing the electrodes directly over metal implants if there is not at least 1 cm of muscle fibre in between. However placement
on the nearest muscle is possible. If in doubt, seek medical advice.
SAFETY INFORMATION
4
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 6

5
SAFETY INFORMATION
• Precautions should also be taken if muscle stimulation occurs during heavy menstruation or in the same month as the insertion of
an IUP (inter-uterine pessary, e.g. coil). The same applies to the period (6 weeks) after giving birth. We recommend that stimulation
is only applied around the abdominal or lower abdominal region following medical approval.
• Medical advice must be obtained before applying stimulation on recent fractures or to parts of the body where feeling is limited.
•
In all cases, ensure that stimulation does not exceed the patient’s tolerance level.
• When repositioning electrodes during treatment, always turn the intensity to minimum or pause the unit.
•
Lead pins and electrodes must not be connected to other objects.
• When the cables are attached to the electrodes, ensure that the lead pins are fully inserted into the electrode sockets. Ensure
that no metal is visible.
• The device should be kept safely out of the reach of children.
•Simultaneous connection of a patient to high frequency surgical equipment may result in burns at the site of the stimulator
electrodes, and possible damage to the stimulator.
• Operation in close proximity to shortwave or microwave therapy equipment may produce instability in the stimulator output.
• Electronic monitoring equipment (such as ECG monitors and ECG alarms) may not operate properly when the stimulator is in use.
• It may not be appropriate to use MediStim XP on a person at the same time as other equipment. You should check suitability
before use.
• The MediStim XPunit should be used only for its intended purpose and in the manner described in this manual. You should also
use only those electrode positions indicated by your clinician.
• A small number of isolated skin reactions have been reported, including allergies and acne.
• Stimulation should not be applied until the cause of the pain is identified and a precise diagnosis rendered.
• To avoid infection electrodes may only be used by a single individual.
• TENS is not intended to treat psychosomatic illness.
• TENS primarily treats symptoms by suppressing pain, which in turn serves as a protective mechanism.
•
This device can deliver current densities in excess of
2mA/cm2when used at a high intensity with small electrodes. See “Technical
Information” for more details.
• The long-term effects of continuous electrical stimulation have not been investigated.
If any irritations, skin reactions, over-sensitivity or other side effects occur, please contact BMR Ltd In such cases stop use immediately.
Irritations can usually be reduced by changing the position of the electrodes. Note, however, that a slight reddening of the skin is
quite normal under the electrodes during and for a short time after treatment.
English
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 7

Please do not use the MediStim XP unit in the following way:
• Stimulation must never be applied transcerebrally (across or through the head), directly on the eyes, covering the mouth,
transthoracicly (electrodes placed on the chest and the upper back or crossing over the heart - as such an application of electrical
current into the heart may cause arrhythmia), or on the front of the neck (especially the carotid sinus nerves).
• Do
not use the MediStim XPunit with the electrodes positioned on injection sites (of medications/
drugs), such as hormone
treatment sites.
• Do not use while driving or operating machinery.
•
MediStim XPmust not be used with any other unit that delivers electrical current to the body (e.g. interferential or another
muscle
stimulator).
General description of MediStim XP
MediStim XP is a battery operated, two-channel Neuromuscular Electrical nerve Stimulatior (NMES) intended for the re-education
and strengthening of atrophied muscle.
MediStim XP also has a Transcutaneous Electrical Nerve Stimulation (TENS) program for the treatment of acute pain.
Based on the principals of NMES and TENS MediStim XP sends short electrical impulses through the surface of the skin via adhesive
electrodes.
Nine treatment programs are available for selection. See Program information on Page 17 for details.
Your MediStim XP package contains:
1. MediStim XP unit 2. Instruction Manual 3. A 9 volt battery
3. Connecting Leads 5. Adhesive electrodes 6. Device box
2.2 Warranty
Should your unit develop a fault within two years of purchase, neurotech®will undertake to replace or repair the unit and parts found
to be defective with no charge for labour or materials *, provided the unit:
• has been used for its intended purpose and in the manner described in this instruction manual.
• has not been connected to an unsuitable power source.
• has not been subjected to misuse or neglect.
• has not been modified or repaired by anyone other than an approved neurotech agent.
This warranty complements existing national guarantee obligations and does not affect your statutory rights as a consumer.
*excludes electrodes and battery pack.
SAFETY INFORMATION & WARRANTY
6
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 8

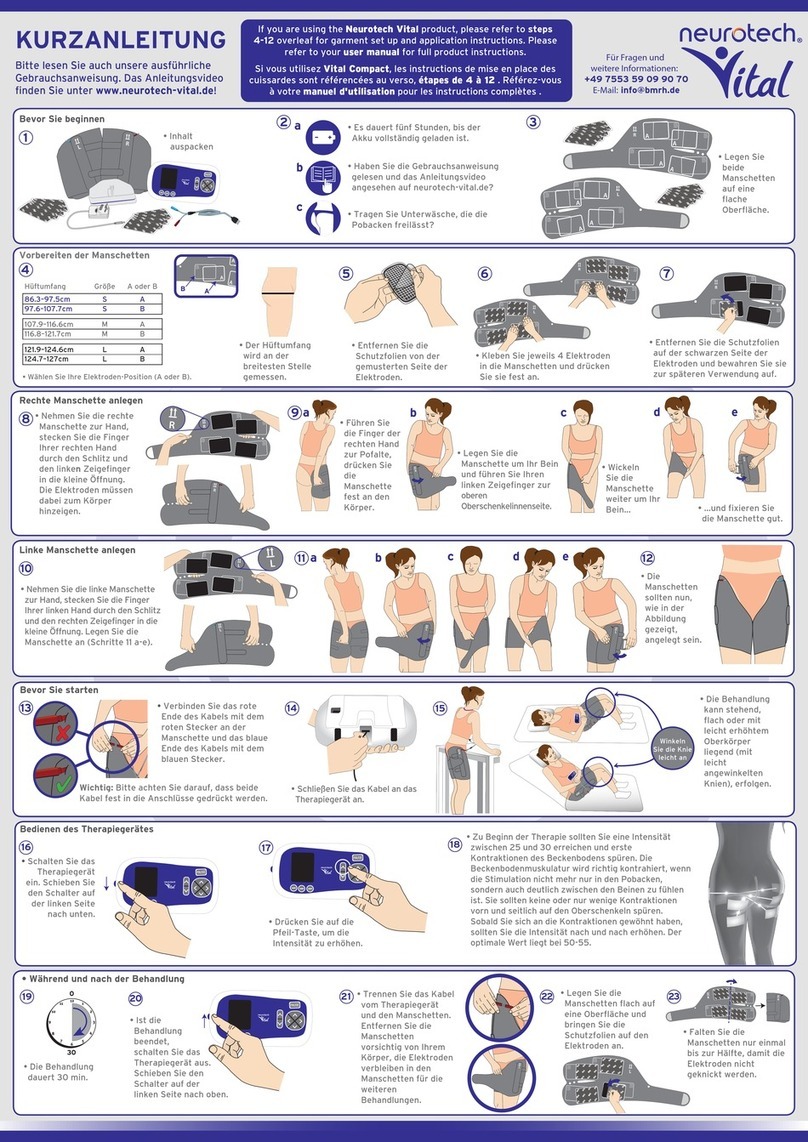
3.0 Description of Unit & Controls
The MediStim XP is easy to use. All keys are controlled by push buttons. The functions are defined
by printed icons on each key (see below). The MediStim XP has a built-in audio indicator which will
emit a raised tone when there is a valid key press and a low tone when an invalid key is pressed.
Keys and Key Functions (Fig. 1)
The MediStim XP has the following controls and functions:
1. On / off (Pause) button ( )
This button switches the unit on and off and is also used to pause the treatment session. You
must press and hold the button (for 2 seconds) to switch the unit off at the end of a treatment.
2. Intensity Controls - Channels 1 and 2 ( / )
Each intensity control controls one channel on the same side of the unit. Pressing the upper
key () during treatment increases the intensity level by a factor of one for that channel.
Similarly, pressing the lower key () can decrease the intensity level by a factor of one. The
numerical intensity indicator displayed on the display changes by one.
3. Program Select Key ( P )
The Program Select key enables the user to select the required treatment program. To change the program hold down the program
selection button P for at least 3 seconds.
4. Lock Key ( )
The Lock key allows the user to lock the intensity controls preventing accidental changes in the intensity level.
5. Trigger Key ( )
Trigger mode: When the key is pressed Trigger mode is enabled and the unit enters a contraction cycle for as long as the key is
pressed. When the key is released the unit enters the relaxation cycle. To return to the programmed contraction/ relaxation cycle,
press any of the intensity keys. The stimulation builds over a 2 second period to the previously set intensity level.
To Reset the Total Treatment Time.
The user must first press the Lock key and then the Program Select for around 3 seconds. A tone will sound and the display will
reset to zero. This function is available only at the start of a treatment session.
1
2
3
4
5
2
7
DESCRIPTION OF IUNIT & CONTROLS
Fig. 1
1. On / off (Pause) button ( )
4. Lock Key ( )
5. Trigger Key ( )
English
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 9

4.0 Description of Unit & Display
Battery Information
The unit is powered by 1 x 9-volt DC battery. The battery compartment is located on the rear of the
unit.
We recommend an alkaline battery. The MediStim XP has an indicator that shows the battery
status. When the battery is nearing discharge, the battery outline will flash. To insert, replace or
check the battery, follow the instructions provided on page 12.
Connecting Leads
Two sockets are positioned at the base of the unit for the insertion of the leads (Fig. 2). The leads are
connected to the electrodes via moulded pins. The electrodes and leads are removable and can be
replaced if necessary. Each lead is a separate channel, one of which is light blue and the other dark
blue. Two plastic moulded pins are found at the end of each lead. They are identified with ‘+’ for the
positive anode and ‘-’ for the negative cathode.
MediStim XP display (Fig. 3)
The MediStim XP has a unique display that gives the user a precise overview of the
battery status, the completed treatment time, contraction/relaxation phases and program
selection.
1.
Lock key is activated and prevents unwanted changes to the intensity level.
2. Load Sense Feature, activated when a poor lead-electrode or electrode-skin connection
is detected.
3. During treatment the intensity bars will rise and fall corresponding to the contraction/ relaxation cycle on each channel.
4. Displays the length of time left/elapsed in the current session in hours, minutes and seconds. For a set treatment time program,
the timer will count down in minutes and seconds. For an open treatment time it will count up from zero in minutes and hours.
5. Battery status indicator, indicates battery power remaining.
6. Clock symbol appears when the Total Treatment Time is displayed and when the clock is counting upwards.
7. Indicates which treatment programme you are running (1 to 9).
8. Trigger mode enabled.
9. Pause indicator, appears when the treatment has been paused.
Fig. 2
DESCRIPTION OF UNIT & DISPLAY
8
Fig. 3
1.
2.
3.
5.
6.
7.
8.
4. 9.
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 10
9
STEP BY STEP TREATMENT GUIDE
5.0 Step by Step Treatment Guide
1.
Using a mild soap and water solution,
clean the skin thoroughly where you will
be placing the electrodes. The electrodes do not adhere well if any dirt, oils,
creams or other cosmetics are still on the skin.
2. Ensure that the device is switched off.
3. Insert, exchange or check battery as described on page 12. The battery should
be exchanged when the 3 bars have disappeared and the battery symbol icon
( ) flashes in the display.
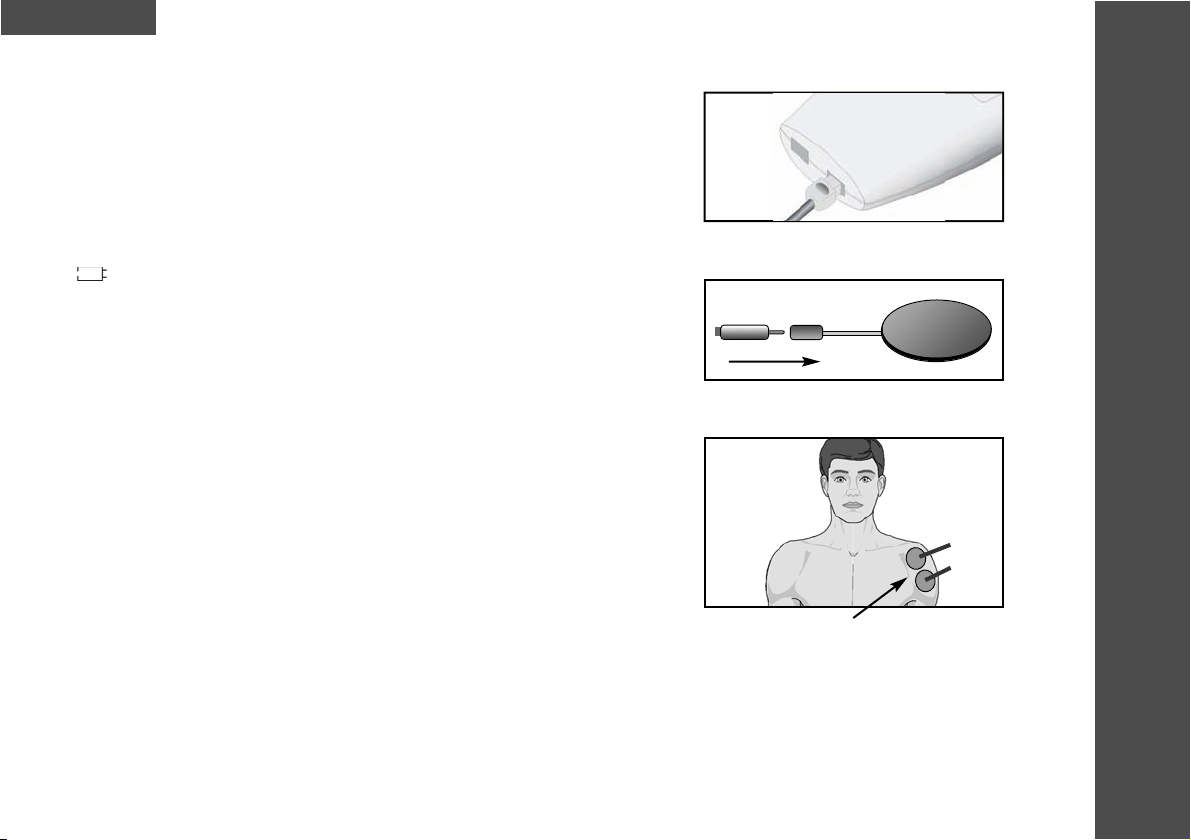
4. The cables supplied with the MediStim XP are inserted into the
sockets on the
underside of the device. The plugs have been designed
so that they click firmly
into place after insertion (Fig. 4). After connecting the leads to the unit, attach
each lead to an electrode (Fig. 5).
5. The MediStim XP is supplied with a set of electrodes. Remove the liners from
the adhesive side of each pad and position the pads correctly. The electrodes
should be handled as stated in the manual.
Please note the following points:
• A clinician must provide instruction on electrode placement and determine
electrode sizes to be used.
• The safety information provided in this manual must be followed.
• The lead pins must be fully inserted into the electrode connector with no metal
pin visible.
• The complete surface of these electrodes should be in contact with the skin
(refer to example in Fig. 6).
• Once the electrodes are attached, you may separate the leads to allow for better
electrode placement.
• The MediStim XP is equipped with a belt clip. You may attach the unit at the waist
by attaching it to a belt. Alternatively, the unit can be hand-held.
( ) flashes in the display.
Fig. 5
Fig. 4 Insert plugs into sockets
Fig. 6 The complete surface of the
electrodes should make contact
with the skin.
English
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 11

6. When the MediStim XP is switched on you hear will a high sound. The screen will display the Total
Treatment Time in hours and minutes for a period of 3 seconds (Display 1). After 3 seconds the screen in
Display 2 will appear.
7. To change the program hold down the program selection button P for at least 3 seconds. The user is
then presented with each available program (1-9) in turn.
Note: You cannot change a program during treatment.
8. Programs 1 - 8 are limited in terms of time (Display 2). Program 9 is not limited in terms of time (Display 3).
9. If you wish to reset the Total Treatment Time press the Program Select and Lock keys simultaneously for
a period of 3 seconds. The Total Treatment Time will reset to zero (Display 4). The maximum Total Treatment
Time is 99 hours and 59 minutes. It will reset back to 00:00 when the maximum treatment time is reached.
10. Slowly begin to increase the intensity on the channel you wish to use, by pressing the corresponding intensity
control. As the intensity is being increased for a particular channel, the stimulus will be felt from the
corresponding electrodes and the intensity bars will rise and fall with the contraction/ relaxation cycles of the
channel being used. The level will be indicated (0 to 99) on the display (Display 5). The treatment timer will
begin once you begin to increase the intensity.
11.
If necessary repeat the process for the other channel. The intensity level from each channel is shown on the
display
.
12. Continue to increase the intensity until the desired level has been achieved. Where more than one channel
is being used, you may increase the intensity completely from one channel before increasing the intensity
from the other.
Display 6 shows the screen during a contraction cycle for a timed treatment program. The Timer displays
minutes and seconds and is counting down. Display 7 shows the screen during a contraction cycle for an open
treatment time program. The timer displays hours and minutes, and is counting up.
Display 1 - Up to 3 sec.
Display 2 - After 3 sec
Display 3
Display 4
10
STEP BY STEP TREATMENT GUIDE
Display 5
Display 6
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 12

11
STEP BY STEP TREATMENT GUIDE
13. Once the desired intensity level has been reached the user can press the lock key to avoid unwanted changes
to the intensity level. If you press the lock key the key symbol ( ) appears (Display 8). To disable the
Lock function, simply press the lock key once again and the key symbol will disappear from the display.
14. If you want to interrupt the treatment session (e.g. to replace the electrodes), briefly press the on/off
(pause) button. The unit issues a beep and the pause icon appears on the display
(Display 9). To deactivate
the pause
function, press the on/off (pause) button again. Then the treatment session is restarted from
where it was paused and the pause icon disappears from the display.
15. The Trigger mode ( ) is possible in Programs 1 – 5, 8 and 9. When the button is pressed the trigger mode
is activated and the unit enters a contraction cycle for as long as the key is pressed (Display 10). When the
key is released the unit enters the relaxation cycle. To return to the programmed contraction/ relaxation
cycle, press any of the intensity keys. The stimulation builds over a 2 second period to the previously set
intensity level.
16. MediStim XP has a load sense function that monitors the connection between the cable/electrode and the
user. When poor skin contact is detected:
•The amplitude bar of the channel being used will flash.
•The warning symbol ( ) will appear flashing on the display (Display 11).
•An audible beep will emit from the unit.
•The treatment session timer pauses.
•The intensity value falls to zero and the intensity increase button is deactivated.
When proper contact is restored, stimulation builds over a 2 second period to the previously set intensity
level.
17. When the treatment is complete, the stimulation will stop automatically.
You will hear a 10 second beep
alerting you that the treatment session
is complete and the display screen will appear as in Display 12.
At this stage the unit should be switched off and all electrodes removed from the body. Replace the
protective liners on the adhesive electrodes until the next session.
Note: The unit power will turn off automatically after 10 seconds.
Display 8 Lock key
activated.
Display 7
!
Display 9 - Pause
activated
Display 11
Display 12
Display 10
( ) is possible in Programs 1 – 5, 8 and 9. When the button is pressed the trigger mode
to the intensity level. If you press the lock key the key symbol ( ) appears (Display 8). To disable the
English
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 13
12
SYSTEM MAINTENANCE
6.0 System Maintenance
The unit should be cleaned regularly using a soft cloth, lightly dampened with soapy water.
Do not allow the interior of the unit or any of the connectors to become wet during cleaning. Do
not use detergents, alcohol, spray aerosols or strong solvents on your unit.
The battery symbol ( ) will appear at all times during operation in the top centre of the display.
When the battery of the MediStim XP is discharging the three bars on the battery symbol
disappear one after another. Once all three bars have disappeared, the battery outline starts to
flash. This means that the battery must be exchanged.
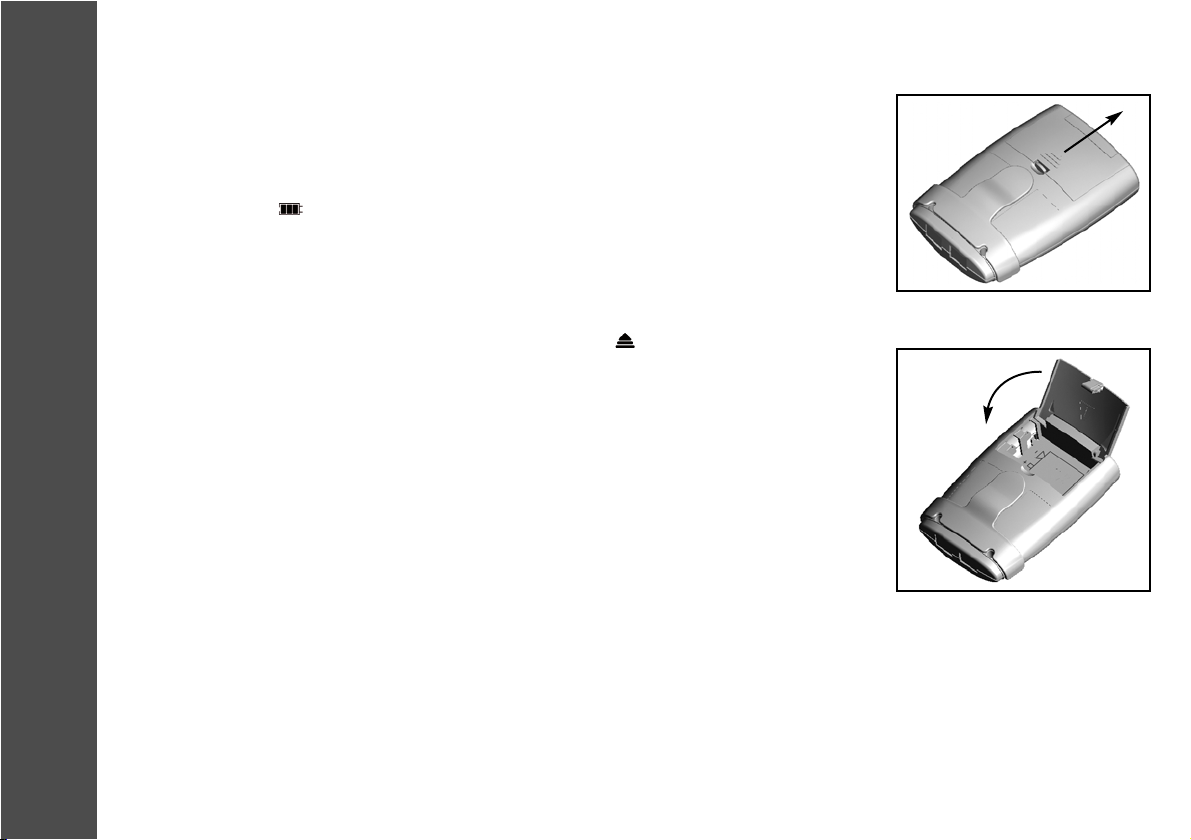
The battery compartment is located on the rear of the MediStim XP unit. In order to open the
battery compartment, insert your thumb into the symbol shown ( ) on the battery compartment
to unlock it and press it forwards. This unlocks the battery compartment.
Now open the cover completely (Fig. 7).
To remove a battery, press firmly against the lower end of the battery and lift it out carefully.
The correct direction to insert the battery is marked by the image of a battery and its connections
in the battery compartment. You need a 9 volt battery.
To close the battery compartment, lower the battery cover and click it into place by applying
slight pressure (Fig. 8).
Note: Keep the battery cover closed when the unit is on.
Fig. 7
Fig. 8
The battery symbol ( )
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 14

13
SYSTEM MAINTENANCE
It is advisable to use a leak-proof battery to help prevent corrosion. We suggest using
alkaline batteries. Never leave a battery in the
unit if it is not intended to be used for a long period of time. If you do, the battery may leak causing damage to the unit. You should
be aware that some batteries sold as ‘leak-proof’ can still release corrosive substances, which may damage the unit. Under no
circumstances should anything other than the correct type of battery be used.
6.1 Accessories
Only electrodes and leads specified by BMR Ltd for use with MediStim XPmay be used. Using other electrodes and leads may
degrade performance levels.
Do not dispose of used electrodes and batteries in household rubbish or in an open flame; dispose of them in accordance with regulations
in your country.
Electrodes wear out over time: If they are dirty or no longer adhere properly, they need to be replaced. Replace the leads if the
sheathing is damaged and exposes the copper wire.
Repair, Service & Modification
Access to the interior is not required for maintenance purposes.
Repair, service and modifications may not be carried out by anyone other than qualified service personnel authorised by BMR Ltd
Do not use the unit if it is defective. Please return it to neurotech. BMR Ltd will not accept any responsibility where the guidelines
and instructions are not followed.
Service and maintenance
A service (safety check) is required at the latest after 24 months according to the manufacturer. For service or repair please send
your electrical stimulation unit to:
neurotech Bio-Medical Research Ltd., Parkmore Business Park West, Galway, Ireland.
English
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 15
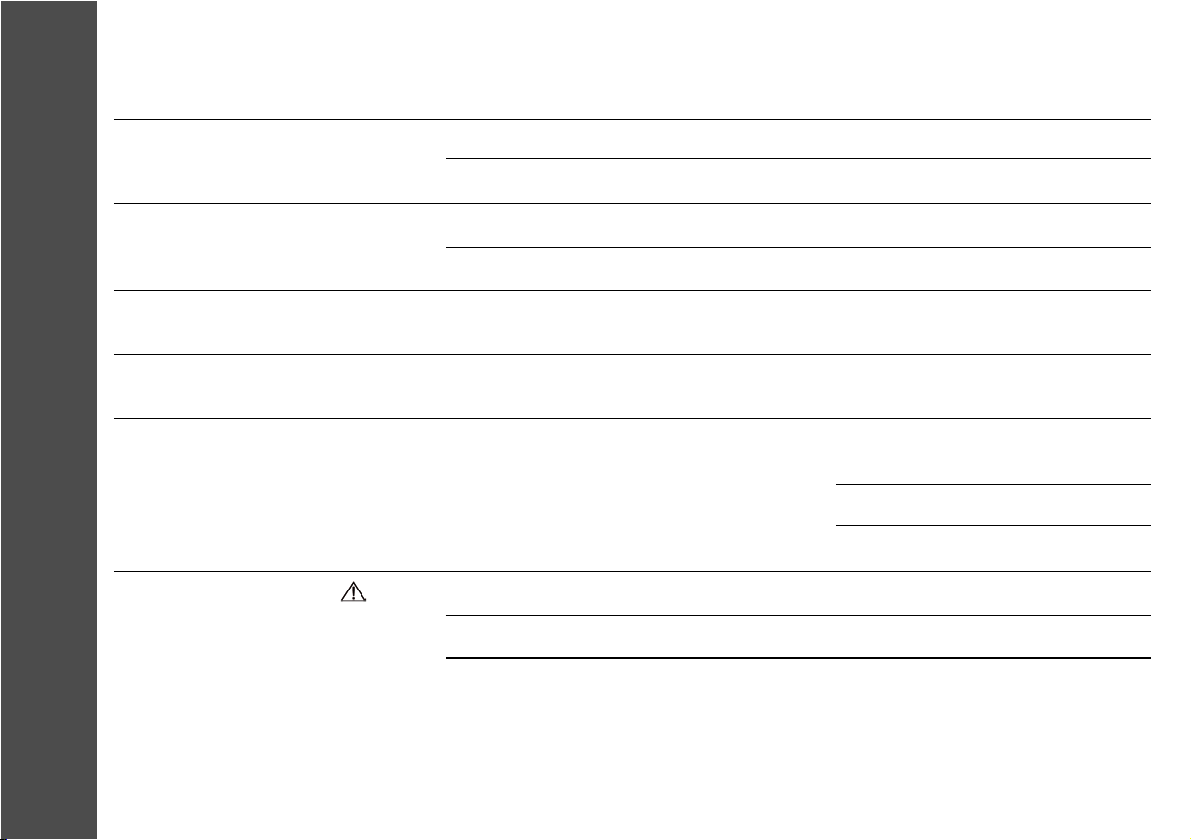
7.0 Troubleshooting
Problem
The display does not come on & there
is no signal from the unit
The unit is switched on but does not
respond to commands
Battery symbol flashing: Ineffective
stimulation
Stimulation received irregularly, only
at a high intensity, or not at all
Increasing intensity causes
unpleasant sensation
Load Sense symbol appears , unit
beeping
Possible Cause
Battery discharged
Battery was incorrectly positioned
Lead not fully inserted
Broken lead
The battery is low
Faulty lead
Check your skin for lotions, pigment marks,
dry marks or other factors that could increase
resistance.
Faulty lead assembly
Electrode faulty
Poor skin/electrode contact
Solution
Replace battery
Remove battery, replace correctly
Remove plug, reinsert
Replace electrode/ lead assembly
Replace the battery
Replace lead
Slowly move electrode to locate area
where stimulus feels strongest
Moisten electrodes
Wash any oils from the skin
Check connections, replace if broken
Replace electrode
Check electrode contact with skin
14
TROUBLESHOOTING
Load Sense symbol appears , unit
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 16

15
TECHNICAL INFORMATION
Nominal output voltage / power
Parameter 500Ω1kΩ1.5kΩ
Output RMS voltage (RMSV) 7.5 V 12.3 V 13.7 V
Output RMS current (RMSA) 15 mA 12.3 mA 9.2 mA
Output frequency 4-99 Hz 4-99 Hz 4-99 Hz
DC Component 0 C 0 C 0 C
Pulse Width 80–400 µs80–400 µs80–400 µs
Current Intensity Range (per pulse) 0–75 mA 0–75 mA 0–75 mA
8.0 Technical Information
General Specifications:
Product Type: 281
No. of Channels: 2
Waveform: Symmetric Bi-Phasic
Environmental Specifications:
Operation: Temperature 0° to 35° C
Humidity 20 to 65 % RH
Storage: Temperature 0° to 55° C
Humidity 10 to 90 % RH
Physical Specifications:
Unit Dimensions: 105 x 71 x 31mm
Weight
• Unit 93 g
• Unit with battery 140 g
XP units are products of BMR Ltd, Parkmore Business Park
West, Galway, Ireland.
Safety Features
Safe start: The intensity is set automatically to zero when
the unit is turned on.
Multiplexing: Pulse delivery to each channel is off-set so
that only one channel is energised at any instant. This
ensures there is no interaction between the electrodes of
each channel.
Electrode area less than 6.5 cm2can cause current densities
in excess of 2m/cm2at maximum intensity. If in doubt, contact
neurotech or your clinician.
English
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 17

16
TECHNICAL INFORMATION
A number of symbols are printed on your unit. Those not already explained are described below:
Power Requirements: 9-Volt, DC Battery (Type 6F22). Inside the battery compartment ‘+’ indicates positive polarity and ‘-’
indicates negative polarity. DC (Direct Current) is indicated by the symbol:
Output RMS Current (RMSA): Stands for the effective current output, which is the root mean square
current measured at a specified resistance.
Output RMS Voltage (RMSV): Stands for the effective voltage output, which is the root mean square
voltage measured at a specified resistance.
Power (P): Maximum power output measured in Watts (W) into a 500Ωload.
Frequency (F): Number of pulses output by the unit per second, measured in Hertz (Hz).
This icon means “Warning, read the accompanying documentation”.
This symbol means type BF applied parts.
SN stands for “serial number”. On the rear of each XP model is the unit’s individual serial number. The letter preceding the serial
number indicates the year of manufacture, where “O” denotes 2009, “P” denotes 2010, etc.
This icon on your XP model shows that the device meets the 93/42/EEC Directive for medical devices.
0366 is the number of the notified body (VDE).
Disposal of device
At the end of the product lifecycle, do not throw this product into the normal household garbage, but bring it to a collection
point for the recycling of electronic equipment.
Some product materials can be re-used if you bring them to a recycling point. By re-using some parts or raw materials from
used products you make an important contribution to the protection of the environment. Please contact your local authorities
if you need more information about collection points in your area.
Waste Electrical and Electronic Equipment can have potentially harmful effects on the environment. Incorrect disposal can
cause harmful toxins to build up in the air, water and soil and can be harmful to human health.
!
0366
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 18
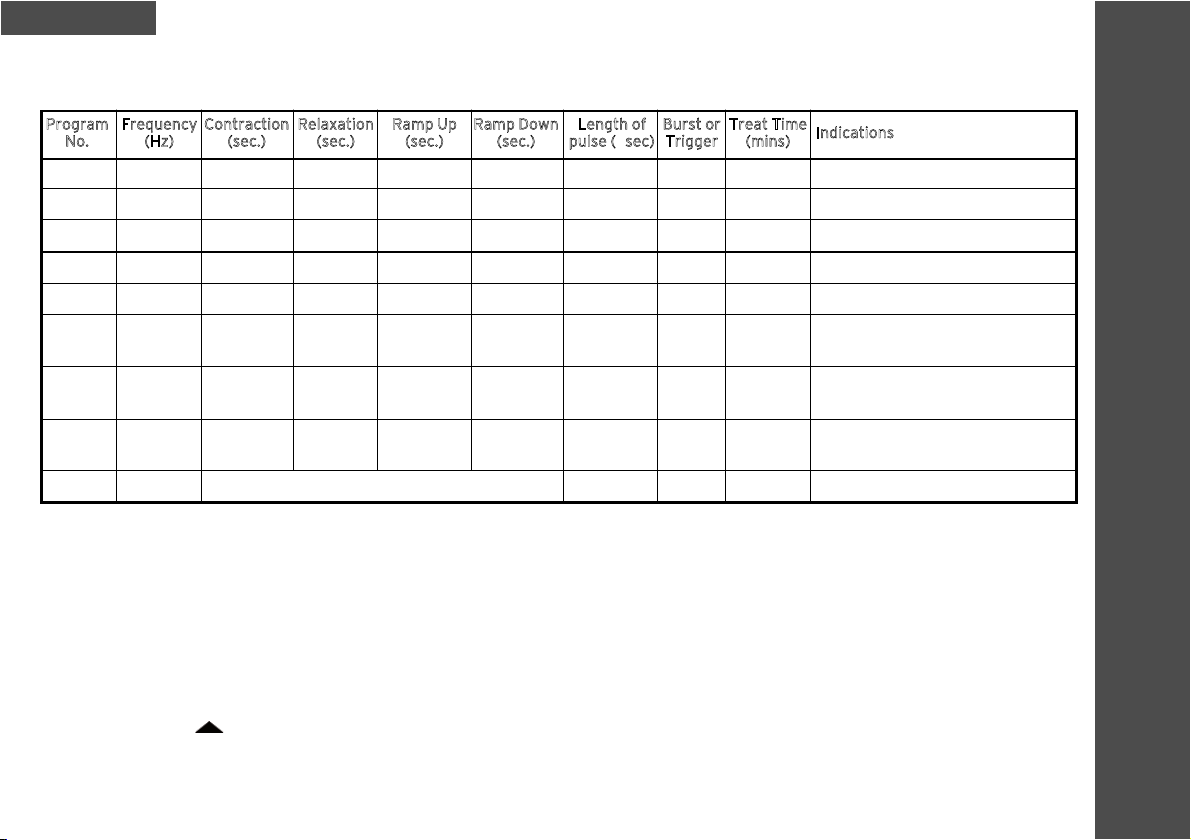
17
PROGRAM INFORMATION
Program
No.
1
2
3
4
5
6
(Note 1)
7
(Note 2)
8
(Note3)
9
Frequency
(Hz)
50
50
50
35
10
ch1: 50
ch2: 10
35
8
4 – 99
Relaxation
(sec.)
5
10
20
5
5
5
5
5
Contraction
(sec.)
5
5
10
5
5
5
5
5
Length of
pulse (µsec)
300
300
400
300
300
300
350
80
150
Treat Time
(mins)
30
30
30
30
30
30
30
30
Open
Indications
Strengthening Rehab
Atrophy Rehab
Sports Strengthening
Neurological Rehab
Early Muscle Activation or Detoning
Tone / Detone
Oedema Management
Facial Palsy
Acute Pain Management
Burst or
Trigger
Trigger
Trigger
Trigger
Trigger
Trigger
None
None
Trigger
Trigger
9.0 Program Information
WARNING: The selection and setting of the program should only be made by the treating clinician.
Note 1: For this program, the output signal sequence is as follows: Channel 1 enters a contraction cycle at frequency of 50Hz for 5
seconds and Channel 2 is off; Channel 1 is off and Channel 2 enters a contraction cycle at frequency of 10Hz for 5 seconds; Both
channels are off for a relaxation cycle of 5 seconds.
Note 2: For this program, the output signal sequence is as follows: Channel 1 enters a contraction cycle for 5 seconds and Channel 2 is
off; Both channels are off for a relaxation cycle of 5 seconds; Channel 1 is off and Channel 2 enters a contraction cycle for 5 seconds;
Both channels are off for a relaxation cycle of 5 seconds.
Note 3: For this program, when the amplitude is at maximum, the value written to the R2R resistor network is less than half the
maximum of 255.
The Trigger mode ( ) is possible in Programs 1 – 5, 8 and 9. When the button is pressed the trigger mode is activated and the unit
enters a contraction cycle for as long as the key is pressed. When the key is released the unit enters the relaxation cycle. To return
to the programmed contraction/ relaxation cycle, press any of the intensity keys. The stimulation builds over a 2 second period to
the previously set intensity level.
Continuous stimulation
Ramp Down
(sec.)
1
1
1.5
1
0.5
0.5
0.5
0.5
Ramp Up
(sec.)
1
1
1.5
1
1
1
1
1
( ) is possible in Programs 1 – 5, 8 and 9. When the button is pressed the trigger mode is activated and the unit
English
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 19

TABLE DES MATIERES
2
TABLE DES MATIÈRES
1.0 Consignes de Sécurité ________________________________________________________________
2.0 Contre-Indications ___________________________________________________________________
2.1 Mesures de précaution et mises en garde _________________________________________________
2.2 Garantie _____________________________________________________________________________
3.0 Description de l’Appareil et des Touches ________________________________________________
4.0 Description de l’Appareil et de l’Écran d’Affichage _______________________________________
5.0 Notice de l’Utilisation Étape par Étape _________________________________________________
6.0 Maintenance de l’Appareil _____________________________________________________________
6.1 Accessoires __________________________________________________________________________
7.0 Recherche et Réparation des Pannes __________________________________________________
8.0 Données Techniques _________________________________________________________________
9.0 Information sur les Programmes ______________________________________________________
3
4
4
6
7
8
9
12
13
14
15
17
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 20

3
1.0 Consignes de Sécurité
Domaine de validité
Les caractéristiques techniques et informations mentionnées dans ce document concernent l’appareil de stimulation musculaire
MediStim®XP, auquel ce manuel est joint. Chaque appareil MediStim XPpossède un numéro de série inscrit au dos de l’appareil.
Toutes les informations et données techniques contenues dans ce document sont la propriété de Bio-Medical
Research Ltd. (BMR
Ltd) et ne peuvent être utilisées et diffusées que dans la proportion et pour l’usage qui auront été acceptés
au préalable par écrit par
l’entreprise.
Clause d’exclusion
Tous les appareils fabriqués et commercialisés par Bio-Medical Research Ltd. sont soumis avant envoi à un contrôle complet de leurs
fonctions et sont minutieusement testés. L’utilisation de ces appareils est cependant externe au domaine de responsabilité de
l’entreprise. BMR Ltd ne prend la responsabilité pour la sécurité, la fiabilité et la fonction de ces appareils que dans la mesure où ils
sont utilisés conformément à ce manuel et aux indications et données qui y figurent. L’utilisateur endosse donc la pleine responsa-
bilité de
toutes les conséquences qui résultent de l’utilisation ou d’un usage non conforme de l’appareil. Les modifications,
réparations ou
autres prestations de services doivent être effectuées exclusivement par les techniciens SAV agréés par BMR Ltd
Ce manuel est un guide destiné aux médecins et personnel hospitalier qui doivent aussi déterminer les zones à traiter et le placement
des électrodes.
L’appareil MediStim XP est conçu par et fabriqué pour :
Bio-Medical Research Ltd., Parkmore Business Park West, Galway, Irlande
Limites
La vente et/ou l’utilisation de ces appareils sont soumises à la législation propre aux différents pays. L’importateur, le revendeur ou
l’utilisateur respectif de l’appareil est tenu de respecter ladite législation.
CONSIGNES DE SÉCURITÉ
Français
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 21

Utilisation
Le MediStim XPpermet une stimulation musculaire et nerveuse selon les principes de l’électrostimulation neuromusculaire (ESNM) et
de la stimulation nerveuse électrique transcutanée (SNET). Il s’agit de courtes impulsions électriques délivrées par l’intermédiaire
d’électrodes placées à la surface de la peau.
Électrostimulation neuromusculaire (ESNM)
L’ESNM peut être définie comme une stimulation électriq u e d u syst ème nerveux périphérique dans laq u e l l e les musc l es so nt stimul é s
soit par l’activation directe des motoneurones dans le nerf périphérique mixte, soit indirectement par des réflexes renforcés.
Stimulation nerveuse électrique transcutanée (SNET)
La SNET est un traitement contre la douleur qui se fonde sur l’application d’une stimulation électrique sur la peau avec stimulation
des fibres nerveuses. La SNET peut être pratiquée de deux manières : soit selon la théorie « pain gate », qui a pour effet de bloquer les
impulsions de douleur envoyées au cerveau, soit par la libération accrue d’endorphines, qui inhibent les phénomènes douloureux.
Indications
• Soulagement en cas de myoplastie
• Prophylaxie ou inhibition de l’atrophie musculaire par inactivité
• Amélioration des symptômes et traitement des douleurs chroniques et aiguës
2.0 Contre-indications
•
Les patients avec des implants électroniques (par exemple pacemaker et défibrilateurs) ne doivent pas utiliser
l’appareil !
2.1 Mesures de précaution et mises en garde
• Prenez systématiquement l’avis du médecin si vous avez les moindres doutes.
• La prudence s’impose pour les patients chez lesquels des problèmes cardiaques ont été diagnostiqués ou sont probables.
• Le conseil du médecin est également indispensable aux patients souffrant de diabète insulinodépendant ou qui sont sous surveillance
en raison d’autres perturbations des fonctions cognitives.
• Les personnes souffrant d’une maladie sévère ou de blessures graves doivent elles aussi consulter un médecin avant toute
stimulation musculaire.
• Pendant la grossesse, dans les cas de cancer ou d’épilepsie, la stimulation musculaire ne présente pas un degré de sécurité suffisant.
• N’appliquez pas sans prendre un avis médical la stimulation à des parties du corps qui ont été récemment soumises à une opération.
• Evitez l’application sur un tissu cicatriciel frais ou sur des plaies ouvertes ou enflammées, tout comme à des endroits infectés par
l’acné ou propices à l’acné, en cas de thrombose ou de toute autre pathologie vasculaire (par exemple veines éclatées ou varices).
• Evitez de placer les électrodes directement au-dessus d’implants métalliques, si ceux-ci ne sont pas au minimum recouverts par 1 cm
de masse musculaire. Il est cependant possible de poser l’électrode sur le muscle le plus proche. En cas de doute, adressez-vous
à un médecin.
• Un avis médical doit également être pris lorsque la stimulation doit être effectuée sur des parties du corps à sensibilité réduite
ou à des emplacements présentant des fractures osseuses récentes.
CONSIGNES DE SÉCURITÉ
4
MediStim XP IM W Europe_PolyStim XP GA 290905.qxd 01/12/2010 12:49 Page 22
Table of contents
Languages:
Other Neurotech Medical Equipment manuals
Popular Medical Equipment manuals by other brands

stemoscope
stemoscope STEMO300 instruction manual

Confycare
Confycare FIX-MT2 Assembly instruction

ulrich medical
ulrich medical Obelisc CS 2931 Series Assembly and disassembly instructions with special cleaning instructions
ivWatch
ivWatch 400 user manual

Planmeca
Planmeca 2D user manual

Nuga
Nuga N5 user manual

Sissel
Sissel Cold Therapy Compression operating manual

Otto Bock
Otto Bock 50R301N Dyneva light Instructions for use

FujiFilm
FujiFilm VisualSonics Vevo F2 user manual

QRS
QRS QRS-101 operating instructions

EKF Diagnostics
EKF Diagnostics DiaSpect Tm user manual

Stryker Medical
Stryker Medical Renaissance Series Maintenance manual