NewTom VG Series User manual

0051
97050869
Rev. 3
28.04.2017
NewTom VGi evo User Manual
EN

2
USER MANUAL
EN
NOTES
This document is provided as a consultation manual intended for the device users.
CEFLA s.c. follows a policy based on the constant development and update of the product. For this reason, it
reserves the right to change the content of this manual without prior notice.
This document can not be modified, copied, reproduced, distributed, saved on magnetic or optical supports, or
published on websites and other on-line services, in full or in part, without the prior written authorisation of CEFLA
s.c.
The original version of this manual is in Italian.
NEWTOM™ VG is a trade mark of CEFLA s.c.
All other products and trade names mentioned in this document are registered marks of the relevant manufacturers.
INFORMATIVE NOTE OF THE MANUFACTURER ON THE MEDICAL DEVICES
The medical device referred to in this manual consists of a scanner and a control, display and calculation unit (Main
Workstation). Such device, as delivered and configured by the production and assistance technical personnel, is an
X-ray device compliant with the safety requirements set forth by the Italian Legislative Decree of 19 September 1994,
no. 626 implementing Directives 89/391/EEC, 89/654/EEC, 89/655/EEC, 89/656/EEC, 90/269/EEC, 90/270/EEC,
90/394/EEC and 90/679/EEC concerning the improvement of the health and safety of workers in the workplace, and
with the essential requirements set forth by the Italian Legislative Decree 24 February 1997, no. 46 implementing
Directive 93/42/EEC as amended, on the medical devices.
The medical device referred to in this manual is an X-ray device compliant with Directive 2011/65/EU on the
restriction of the use of certain hazardous substances in electrical and electronic equipment.
Any tampering with, modification, updating or other change both of hardware1and software2of the device as supplied
and installed by the company (and in the conditions specified in the attached documentation) may partially or totally
compromise the device expected operation. This may also alter the safety features with consequent hazard increase
for patients, operators and surrounding environment.
For this reason, should the user need to modify the device, he/she must request a written authorisation by CEFLA
s.c.
Failure to comply with what is specified in this informative note will null and void the device warranty and the civil
and/or penal responsibility for any consequent damage and/or accident and/or worsening of the patient, operator or
other people health (including the surrounding environment) will be borne by the person who tampered with the
device or his/her legal representative.
1
Adding of a new memory expansion, a new hardware on the connection bus, a printer, the replacement of the
graphic display interface represents an important modification.
2
Including the operative system and the applications already installed upon medical device delivery. Automatic
updates of the operative system, changes to network connection parameters, modification and/or addition and/or
removal of interface software with hardware (device driver) and/or services (e.g. file and printer sharing service)
and/or applications represent an important modification.
ITALIANO

EN
USER MANUAL
3
Contents
1. INTRODUCTION TO THE MANUAL.........................................................................................................................5
1.1. CONTENTS.......................................................................................................................................................... 5
1.2. STRUCTURE ....................................................................................................................................................... 5
1.3. STYLISTIC CONVENTIONS................................................................................................................................ 5
2. SAFETY-RELATED INFORMATION ........................................................................................................................6
2.1. APPLICABLE LAWS, JURISDICTION AND COURT OF JURISDICTION .......................................................... 6
2.2. SYMBOLS ON THE DEVICE ...............................................................................................................................7
2.3. DEVICE SWITCHING ON AND OFF ................................................................................................................... 8
2.4. EMERGENCY SWITCHING OFF ........................................................................................................................ 8
2.5. SAFETY OF PATIENT AND OPERATOR ...........................................................................................................9
2.5.1. PATIENT POSITIONING .............................................................................................................................. 9
2.5.2. DURING THE SCANNING............................................................................................................................ 9
2.5.3. PATIENT GOING OUT OF THE SCANNING AREA ....................................................................................9
2.6. ARTEFACTS AND SCANNING REPETITION.....................................................................................................9
2.7. PROTECTION AGAINST IONIZING RADIATIONS...........................................................................................10
2.8. PROTECTION AGAINST LASER RADIATIONS ...............................................................................................11
2.9. DEVICES CONNECTED TO THE CONTROL CONSOLE ................................................................................12
2.10. MAINTENANCE INTERVAL ..........................................................................................................................12
2.11. APPLIED PARTS ...........................................................................................................................................12
3. DEVICE SAFETY AND MAINTENANCE ................................................................................................................13
3.1. INSTALLATION REQUIREMENTS....................................................................................................................13
3.2. SAFETY GUIDELINES.......................................................................................................................................14
3.3. CHANGES TO THE DEVICE .............................................................................................................................14
3.3.1. LIMITS OF RESPONSIBILITY ....................................................................................................................14
3.4. DEVICE MAINTENANCE ...................................................................................................................................14
3.5. CLEANING AND DISINFECTION ......................................................................................................................17
3.5.1. HYGIENE PROCEDURES FOR PATIENT PROTECTION ........................................................................ 18
3.5.2. STERILISATION ......................................................................................................................................... 18
3.6. TRANSPORT AND STORAGE .......................................................................................................................... 18
3.7. DEVICE DISPOSAL ...........................................................................................................................................19
3.7.1. INFORMATION FOR DEVICE OWNER .....................................................................................................19
3.7.2. INFORMATION FOR COLLECTION / DISPOSAL / RECOVERY FACILITIES..........................................19
4. STARTING PROCEDURES....................................................................................................................................20
4.1. INTRODUCTION TO THE SYSTEM..................................................................................................................20
4.1.1. INTENDED USE.......................................................................................................................................... 20
4.1.2. INDICATIONS FOR USE ............................................................................................................................ 20
4.1.3. IMPROPER USE......................................................................................................................................... 21
4.1.4. FUNCTIONING ........................................................................................................................................... 21
4.2. OPERATION PRINCIPLE ..................................................................................................................................22
4.3. OVERVIEW ........................................................................................................................................................ 22
4.4. SCANNER .......................................................................................................................................................... 23
4.4.1. OPERATOR CONSOLE AND CONTROLS................................................................................................ 23
4.4.2. SCANNER MOVEMENT.............................................................................................................................24
4.4.3. CHINREST SYSTEM MOVEMENT ............................................................................................................24
4.4.4. INFORMATION MENU ............................................................................................................................... 24
4.4.5. SERVICE MENU .........................................................................................................................................25
4.4.6. OTHER FUNCTIONS.................................................................................................................................. 25
4.4.7. X-RAY EMISSION.......................................................................................................................................26
4.5. CONTROL BOX .................................................................................................................................................26
4.5.1. MAIN SWITCH ............................................................................................................................................ 26
4.5.2. INPUT PANEL.............................................................................................................................................26
4.6. STANDARD ACCESSORIES............................................................................................................................. 27
4.6.1. X-RAY EMISSION REMOTE CONTROL....................................................................................................28
4.7. CABLES..............................................................................................................................................................29
4.8. STARTING THE SYSTEM .................................................................................................................................29
4.9. SYSTEM SWITCHING OFF ............................................................................................................................... 29

4
USER MANUAL
EN
5. PRELIMINARY OPERATIONS............................................................................................................................... 30
5.1. DAILY CHECK ....................................................................................................................................................30
5.2. BLANK ACQUISITION........................................................................................................................................31
5.2.1. BLANK ACQUISITION INVALIDATION ......................................................................................................32
5.3. BEAM LIMITER TEST ........................................................................................................................................32
5.4. LOWERING THE ROTATING ARM COMPLETELY ..........................................................................................33
6. SCANNING ............................................................................................................................................................. 34
6.1. SCANNING A PATIENT .....................................................................................................................................35
6.1.1. PREPARING THE PATIENT.......................................................................................................................35
6.1.2. CRANIOSTAT USE .....................................................................................................................................36
6.1.3. POSITIONING THE PATIENT AND STARTING THE SCANNING ............................................................38
6.1.4. SPECIAL CONSIDERATIONS FOR CHILDREN AND SMALL PATIENTS ...............................................40
6.2. SCANNING A PROSTHESIS .............................................................................................................................41
6.2.1. PRELIMINARY OPERATIONS....................................................................................................................41
6.2.2. POSITIONING THE PROSTHESIS ............................................................................................................41
7. QUALITY CONTROL .............................................................................................................................................. 42
7.1. PHANTOM POSITIONING .................................................................................................................................42
7.2. IMAGE EXAMPLES............................................................................................................................................43
7.3. SAVING THE PHANTOM ANALYSES ...............................................................................................................43
8. TROUBLESHOOTING............................................................................................................................................ 44
9. APPENDIX A: TECHNICAL SPECIFICATIONS..................................................................................................... 45
10. APPENDIX B: COMPATIBILITY............................................................................................................................. 65
11. APPENDIX C: DEVICE LABELS ............................................................................................................................ 66

EN
USER MANUAL
5
1. INTRODUCTION TO THE MANUAL
1.1. CONTENTS
This manual has been conceived as a consultation document to provide information and instructions on the use of
the NewTom™ VG series device, “NewTom VGi evo” model.
The routine software operation set for this device (scanning, data processing, reporting and document management)
and the use instructions for the operator are dealt with in the “Acquisition Operations with NewTom VGi evo” annex to
the "NNT User Manual" document.
The “USER MANUAL” of the device, "NNT User Manual" and “Acquisition Operations with NewTom VGi evo” should
be read and understood in every part before starting to use the device.
We recommend keeping this manual together with the other documentation and using it as a guide if new personnel
must be trained on the use of the device.
1.2. STRUCTURE
The “User Manual” is divided into the following chapters:
Chapter 1 - “INTRODUCTION TO THE MANUAL”:
Provides information on the contents, the structure and the conventions used in this document.
Chapter 2 –“SAFETY-RELATED INFORMATION”:
Includes information concerning the operator and patient safety and fundamental use procedures of the equipment.
Chapter 3 –“DEVICE SAFETY AND MAINTENANCE”:
Contains information on the safety requirements and the device maintenance operations.
Chapter 4 –“STARTING PROCEDURES”:
Provides a general description of the system and its main parts.
Chapter 5 –“PRELIMINARY OPERATIONS”:
Explains the procedure for a correct device initialization.
Chapter 6 –“SCANNING”:
Explains the process to position and scan a patient.
Chapter 7 –“QUALITY CONTROL”:
Explains the procedure for a correct Quality Assurance process.
Chapter 8 –“TROUBLESHOOTING”:
Provides a list of malfunctions and possible solutions.
APPENDIX A: TECHNICAL SPECIFICATIONS
APPENDIX B: COMPATIBILITY
APPENDIX C: DEVICE LABELS
1.3. STYLISTIC CONVENTIONS
Important safety-related information and notes are indicated in the manual as follows:
HAZARD:
Informs about the presence of a potential hazard that may lead to personal injuries or even death.
WARNING:
Warns about the presence of a potential hazard that may damage the device.
NOTE:
Provides further information not concerning the safety of the device, the patient and the operator.

6
USER MANUAL
EN
2. SAFETY-RELATED INFORMATION
This chapter provides safety-related information the operator must become familiar with before using the device.
To ensure the patient and the operator safety, always follow the instructions provided herein, especially as far as
functional tests, electric and mechanic safety and X-ray emission protection are concerned.
In this regard, refer to chap. 3 - “DEVICE SAFETY AND MAINTENANCE"and Chap. 6 - “SCANNING".
WARNING:
All operators must be familiar with the operative and environmental features of the system and
know the procedures to be followed in case of hazard and for the emergency switch-off.
2.1. APPLICABLE LAWS, JURISDICTION AND COURT OF JURISDICTION
Strictly follow all requirements on device installation, maintenance and use. Refer to the local legislation if it is more
severe than the prescriptions contained in this manual.

EN
USER MANUAL
7
2.2. SYMBOLS ON THE DEVICE
The table below provides a description of the symbols on the device labels:
Symbol
Standard
Description
IEC 60417-5010
On / Off (pressure-pressure)
~
IEC 60417-5032
Alternating current
ISO 7000-0434A
Warning
ISO 7010-W001
General warning signal
IEC 60878
ISO 3864-B.3.6
Warning: hazardous voltage.
IEC 60417-5019
Protective earth.
N
IEC 60445
Connection point of the neutral wire of permanently installed equipment.
L
IEC 60445
Connection point of the line wire of permanently installed equipment.
IEC 60417-5841
Applied part of type B, protected against direct and indirect contacts.
IEC 60878-5909
Ionizing Radiations.
Directive
2012/19/EU
Disposal of WEEE (Waste from Electrical and Electronic Equipment).
0051
Directive
93/42/EEC as amended
EC marking.
EN 980:2008
Serial number.
EN 980:2008
Date of manufacture.
EN 980:2008
Manufacturer.
ISO 7000-1641
Operating instructions.
ISO 7010-M002
Refer to the instruction manual.

8
USER MANUAL
EN
Symbol
Standard
Description
IEC 60417-5638
Emergency stop.
Original symbol required
by IEC 60601-1 par. 9
Head collision hazard.
To avoid any collision, refer to par. 6.1.2 - "Positioning the patient and
starting the scanning" to position the patient in the correct way.
2.3. DEVICE SWITCHING ON AND OFF
The device must be switched on and off as specified in the procedures indicated in par. 4.8 and 4.9.
2.4. EMERGENCY SWITCHING OFF
The device is provided with 3 emergency switch-off buttons. One is located on the front panel, near the motor-driven
chinrest system, the second one near the main switch on the control box and the third one on the operator table.
1- Device emergency buttons.
Switching off the device by pressing an emergency button causes the immediate interruption of the emission and all
the motor-driven movement functions of the device.
WARNING:
The emergency switching off must be used exclusively in case of hazardous situations, i.e.:
•The X-ray source does not stop the emission.
•Situations that can injure people, harm the environment or damage the device.
•Conditions in which the system indicates an emergency situation.

EN
USER MANUAL
9
2.5. SAFETY OF PATIENT AND OPERATOR
Work following the correct procedures and position the patient correctly to avoid risks for the patient and the involved
operators.
Pay special care in case of debilitated people or with traumas.
2.5.1. PATIENT POSITIONING
Make sure the patient is correctly positioned in the scanning area, with the head on the chinrest and that no other
part of the body can touch the device or risk to be squeezed during the positioning and the examination.
Make sure that patient's clothes and hair can not remain entangled.
Perform the same check for any catheters, breathing tubes or ECG (Electrocardiography) cables.
Before starting any device movement check that the patient is in the correct position and that there are not obstacles
to the device movements.
Refer to par. 6.1.3 - "Positioning the patient and starting the scanning" and to the attached document “General
guidelines for use of the protocols of New Tom VGi evo”.
2.5.2. DURING THE SCANNING
During the device movement and the patient scanning process NEVER leave the system without a supervisor.
Always keep the patient monitored for the entire scanning duration.
WARNING:
NEVER use the device without the operator supervision.
NOTE:
Consider the implementation of an audio/video communication system between the operator and
the patient in case the operator controls the device from a protected and remote area.
2.5.3. PATIENT GOING OUT OF THE SCANNING AREA
At the end of the examination or after the emergency button has been pressed, it is possible to allow the patient to
leave the scanning area without waiting for the rotary arm to return to the initial position.
2.6. ARTEFACTS AND SCANNING REPETITION
A scanning process must be repeated ONLY if there are important artefacts on a patient's image or if the patient
position has clearly changed during the scanning.
10
USER MANUAL
EN
2.7. PROTECTION AGAINST IONIZING RADIATIONS
WARNING:
NewTom VGi evo is an X-ray device and as such it exposes patients and operators to the risk
deriving from ionizing radiations.
It must be used in compliance with the safety standards set forth by the radiological protection
standard in force in the country of use.
WARNING:
NewTom VGi evo must not be used for routine or screening examinations. For such purposes,
consider other diagnostic equipment.
The imaging examinations performed on each patient must be justified in order to prove that they
provide more benefits than risks.
Strictly follow the applicable radiological protection standards and any prescription provided by a Qualified Expert.
Operator
The operator must follow the examination from a control work station according to the prevailing laws; nobody is
allowed to remain near the patient during the examination.
In case a patient has a panic reaction that requires the intervention of the operator during the examination, the
operator shall wear suitable protection clothes and equipment as defined by the national and local standard.
WARNING:
Never remain near the device during the emission.
Patient
The user is responsible for protecting the patient from useless exposure.
WARNING:
Consider the use of a leaded apron to protect the patient from diffuse radiation.
WARNING:
When prescribing X-ray examinations to pregnant women or women that could be pregnant,
carefully consider the possible radiation consequences on the fetus. When possible, avoid
radiation to a fetus.
WARNING:
Consider the possibility to use a leaded apron with collar for thyroid to protect the patient from
diffuse radiation.
WARNING:
Possible negative interaction of CT x-rays with implantable active and worn active medical
devices.
Contact the manufacturer of such devices for further information.
Emission view devices
The emission status is clearly identified by:
1. A signal on the display as shown below. Such signal is displayed only after the X-ray emission is started by
pressing START on the keyboard or using a mouse (refer to chap. 6 ”Scanning”) and remain visible for the entire
scanning duration.

EN
USER MANUAL
11
2. A signal on operator console as shown below. Such signal is displayed on the operator console only after the X-
ray emission is started by pressing START on the keyboard or using a mouse (refer to chap. 6 ”Scanning”) and
remain visible for the entire scanning and/or emission duration.
WARNING:
If the emission signals are active when the X-ray emission control has not been started, if they are
not active when the emission has been started or if the emission is not interrupted at the end of
the preset time, turn off the system immediately and contact the technical service.
2.8. PROTECTION AGAINST LASER RADIATIONS
The device is provided with a double laser to correctly position the
patient. The laser radiation comes out of two holes on the front cover.
The vertical line indicates the central sagittal plane of the
reconstituted volume. The horizontal line indicates the central axial
plane of the reconstituted volume.
WARNING:
Do not stare at the laser ray, do not look at it directly with optical instruments and avoid the direct
exposure. The ray can cause permanent eye damage.
WARNING:
Keep a distance of at least 40 cm between the eyes and the laser emission point when the laser
ray is active.
If necessary consider the use of suitable protection goggles.
WARNING:
Failure to comply with the prescriptions and procedures described herein may lead to a
dangerous exposure to radiations.

12
USER MANUAL
EN
2.9. DEVICES CONNECTED TO THE CONTROL CONSOLE
Any computer, monitor, printer, mouse, keyboard and any other device connected to the device control workstation
MUST be compliant with the ISO and/or IEC and/or EN and/or local standards.
Moreover, the workstation must be compliant with the IEC 60950-1 standard.
For further information contact the Manufacturer.
NOTE:
The Manufacturer is not responsible for problems and/or malfunctions of parts and/or components
not approved by itself and not installed by qualified technical personnel acknowledged by the
manufacturer.
Never eat/drink or leave beverage/food near the device and the console.
2.10.MAINTENANCE INTERVAL
Make sure that the maintenance operations described in par. 3.4 - “Device maintenance" are carried out.
2.11.APPLIED PARTS
The parts that, during standard use, necessarily come into contact with the patient in order for the device to carry out
its functions correctly, are: chinrest, bite piece and hygienic protections, headrest, handles and nose protections.
The non-applied parts that may come into contact with patient are the external covers and the chinrest structure.

EN
USER MANUAL
13
3. DEVICE SAFETY AND MAINTENANCE
This includes information on device and environment safety. It also provides general information and procedures
concerning the system maintenance.
The user is responsible for a correct use of the system, in compliance with the instructions and procedures provided
in this manual. In particular, the operator must observe the following instructions:
•The device can be used exclusively by authorised personnel, trained on the machine use and the protection from
radiations. Said personnel must also know the standards that regulate the use of X-ray devices.
•The device must never be used in case of evident electric, mechanic or radiological malfunctions. In particular, it
must never be used if the warning or emergency switch-off devices do not work properly.
3.1. INSTALLATION REQUIREMENTS
The system must be used in rooms used for medical purposes in compliance with the recommendations of a
Qualified Expert.
The equipment must never be exposed to acids, corrosive agents, salt and rain.
Operating temperature:
from +10° to +35° (Celsius)
Operating humidity conditions:
min 10%, max 85% (non-condensing)
Altitude:
≤ 3000 m
Pressure:
710 –1060 hPa
Pollution degree:
2
CTI (“comparative tracking index”):
IIIb
Minimum dimension requirements of the installation room: 2 x 2.5 x 2.5 m.
The equipment must be installed on a horizontal surface.
In case of use with seated patient, make sure that the chair backrest is not higher than 75 cm.
The supply line must be arranged according to the prevailing laws and to the instructions provided in the "Service
Manual".
Do not use temporary electric connections like reduction units, extensions, multiple sockets for the connection to the
PC network or to other peripheral devices.
The equipment must be connected to the electric system in a permanent way according to the prescriptions provided
in the "Service Manual".
The medical environment in which the device is installed must be designed by an expert in ionizing radiation
protection as set forth by the prevailing national and local laws. The prevailing national and local laws will define the
rules to be followed to design the signals to be applied to the system.
WARNING:
Never move the device after its installation as this may lead to dangers for people, the device and
the environment.
The device must be connected exclusively with peripheral units, computers and cables compliant
with the manufacturer's specifications.
WARNING:
Make sure the device is connected to an electric line with protective earth.
NOTE:
The computer must be installed outside the patient area.
The connectors connected to the computer cables must be used exclusively for the connection to
the computer.
Such connectors must be handled by authorised and qualified personnel only.

14
USER MANUAL
EN
3.2. SAFETY GUIDELINES
The device is not protected against liquid and spray penetration. The penetration of liquids can damage the electric
and electronic components and generate hazardous situations for the patient, the operator and the environment.
The device safety systems do not reduce the fire-fighting protections installed in the room where the device is used.
•Electrostatic discharges
Electrostatic discharges can damage the machine electronic components. As a consequence, the floor of the room in
which the device is installed should be made of antistatic materials.
•Fire-extinguishers
CO2 fire-extinguishers should be installed in an area easy to be reached.
•X-ray warning lamp
The user has the possibility to install an X-ray warning lamp to be used to know both if the X-ray source is ready and
if the X-ray emission is active.
•Switches on doors
The user has the possibility to install an external switch to stop the emission (usually installed on access doors of the
room where the device is used).
•Electromagnetic compatibility
For information about the electromagnetic compatibility, refer to APPENDIX A - “Technical Specifications”.
3.3. CHANGES TO THE DEVICE
Any change or update of the system must be compliant with the applicable laws.
WARNING:
It is forbidden to open or tamper with the device with any tool.
Any non-authorised change to the system (hardware and software) is forbidden and may
compromise the correct device operation, cause breakages and/or accidents with consequent
possible damages to the patient, the operator and the device.
3.3.1. LIMITS OF RESPONSIBILITY
The manufacturer is not responsible for the safety, reliability and performance features in the following cases:
•The installation, maintenance and any change, repair and/or update are not performed by personnel authorised
by the manufacturer or the distributor.
•The spare parts have not been approved by the manufacturer or the distributor.
•The environment conditions are not compliant with the requirements described in this manual, the requirements
of the applicable laws and the recommendations of a qualified expert.
•The device is not used as described in this manual.
3.4. DEVICE MAINTENANCE
Any change or update of the system must be compliant with the applicable laws.
WARNING:
Always turn off the device before performing any maintenance operation!
WARNING:
None of the internal parts of the equipment can be repaired. Never remove the equipment covers.
EN
USER MANUAL
15
WARNING:
The only part that can be repaired by the user is the device input fuse, located near the switch-on
panel, on the control box side.
The spare fuse must be compliant with the manufacturer's specifications.
WARNING:
To ensure the protection against fire, replace only fuses with others of the same type and range.
•Ordinary maintenance
The ordinary maintenance is required to ensure the correct device operation as well as the safety of the patient,
the operator and of third parties.
The device must be exclusively repaired and maintained by personnel authorised directly by the manufacturer or
the distributor. All components of the system must be checked and, if necessary, replaced by qualified personnel.
WARNING:
If the NewTom VGi evo unit has not been used to scan patients for longer than three months,
please proceed with the x-ray source forming process (see “Acquisition Operations with
NewTom VGi evo” annex to the "NNT User Manual" document).
•Hazardous cleaning agents
Some cleaning agents should be avoided to prevent negative consequences on the device and people (see "3.5
Cleaning and disinfecting").
•Preventive maintenance
Check the computer-scanner interface cables, the control computer-box and the relevant power supply cables at
regular intervals. Check the connection cable to the computer, the monitor, the keyboard, the mouse and the
printer according to the manufacturer instructions.
•Component and accessory storage
Components and accessories must be stored and handled with care.
Any provided components and accessories must be stored and handled in compliance with the relevant technical
specifications.
•Malfunctions
In case the system does not work as described in this manual, contact the technical service immediately.
•Maintenance contract
The device should be checked at regular intervals: contact the manufacturer or the distributor to discuss about a
maintenance contract.
•Check-list of the system checks
The following check-list indicates the recommended time intervals of the various system checks.
For further information contact your local distributor.

16
USER MANUAL
EN
Manager
Component
Activity
Time interval
Routing testing
User
Global system
QA Phantom check
Weekly
Local Support Service
Error log
Check
12 months
Every external part
Damage check
12 months
Emergency buttons
Emergency test
12 months
Electrical functioning
Check
12 months
Mechanical functioning
Check
12 months
Other test depending on local regulations
Radioprotection expert
or other qualified person
depending on local
regulations
Global system
Radiological test
conforming to the local
regulation concerning X-
ray medical electrical
equipment.
This tests in not in charge
by user or local support
service but may be
established by local
regulations.
Radiological test
conforming to the local
standard
EN
USER MANUAL
17
3.5. CLEANING AND DISINFECTION
WARNING:
Always turn off the device before performing any cleaning operation
WARNING:
Cleaning is the first step of any disinfecting process. Physically scrubbing with detergents and
surface-active substances and rinsing with water removes a considerable amount of micro-
organisms. If a surface is not clean first, the disinfecting process cannot be successful.
If a surface cannot be adequately cleaned, it should be covered with barriers.
The outer parts of the equipment must be cleaned and disinfected using a product for hospital use with indications for
HIV, HBV and tubercolocide (medium-level disinfectant) specific for small surfaces.
The various drugs and chemical products used in dental surgeries may damage the painted surfaces and the plastic
parts. Researches and tests performed show that the surfaces cannot be fully protected against the harsh action of
all products available on the market. We therefore recommend protecting with barriers whenever possible.
The harsh actions of chemical products also depend on the amount of time they are left on the surfaces. It is
therefore important not to leave the product on the surfaces longer than the time specified by the manufacturer.
We recommend to use a specially formulated medium-level disinfectant, STER 1 PLUS (CEFLA S.C.), which is
compatible with coated surfaces, plastic parts and uncoated metal surfaces. As an alternative, we recommend to use
products containing:
- 96% ethanol. Concentration: maximum 30 g per 100 g of disinfectant.
- 1-Propanol (n-propanol, propyl alcohol, n-propyl alcohol). Concentration: maximum 20 g per 100 g of disinfectant.
- Combination of ethanol and propanol. Concentration: the combination of the two should be maximum 40 g per
100 g of disinfectant.
WARNING:
- Do not use products containing isopropyl alcohol (2-propanol, iso-propanol).
- Do not use products containing sodium hypochlorite (bleach).
- Do not use products containing phenols.
- All products must be used as directed by the manufacturer.
- Do not mix the STER 1 PLUS disinfectant with other products.
- Do not spray the selected products directly on the surfaces.
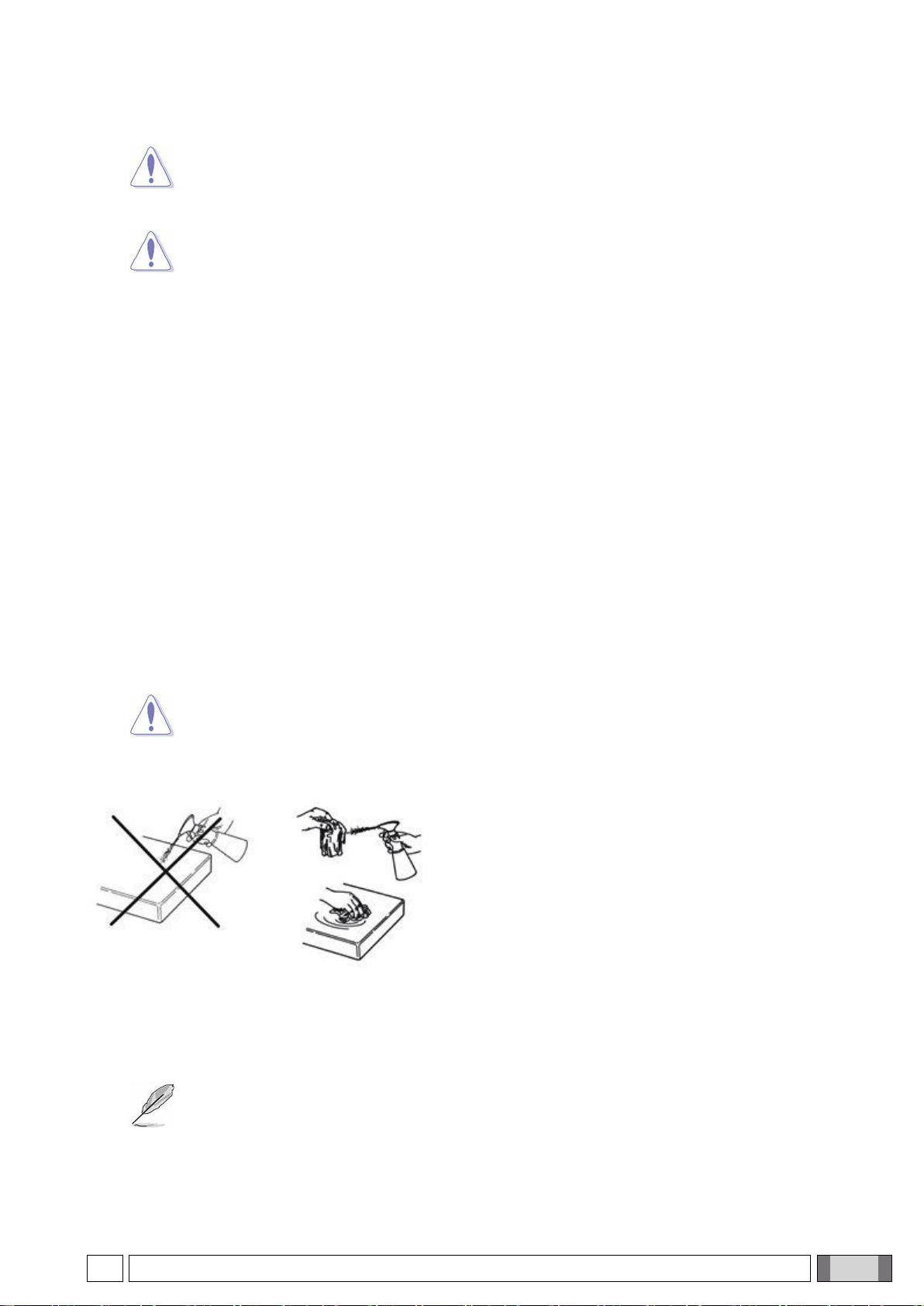
Clean and disinfect with disposable non-abrasive paper (avoid
using recycled paper) or sterile gauze.
-Turn off the equipment prior to cleaning and disinfecting the
external parts.
-All materials used to clean and disinfect must be thrown
away.
-Computer and peripheral devices
Follow the manufacturer's instructions to clean the computer and the peripheral devices. If such instructions are
not available, refer to the instructions provided in the previous paragraph.
NOTE:
Contact the local distributor for further information about the device safety and maintenance.

18
USER MANUAL
EN
3.5.1. HYGIENE PROCEDURES FOR PATIENT PROTECTION
Disposable hygienic protections are the main protection means against cross contamination between patients. In
order to prevent the transmission of infectious diseases between patients, it is essential to always use disposable
protections. Disposable protections are class I medical equipment and cannot be replaced with other protections
having lower specifications.
Disposable protections must comply with standards ISO 10993-1 on biocompatibility and be approved by control
bodies where required (e.g. FDA, CE).
Always replace bite disposable hygienic protections before positioning a new patient.
Disposable hygienic protections must be stored in a dry and clean area and must not be exposed to direct sunlight or
UV radiation.
Bite and chin rest can be disinfected by soaking them in a cold sterilising liquid. For sterilisation of such parts, follow
the instructions provided by the sterilising product supplier.
Cover with disposable protections all components that will be in contact with dental personnel's hands and might be
contaminated by indirect contact with the mouth of the patient. In particular, pay attention while handling equipment
control console, mouse and Personal Computer keyboard.
Before positioning the patient for a radiological examination, always cover the bite with a new (non-sterile) plastic
protection in order to prevent cross contamination.
Note for Canada users: ask your dental distributor for hygienic protections with suitable size and marketed in Canada
according to the local laws.
In compliance with the provisions of Health Canada, bite protections are Class I equipment supplied by authorised
distributors as per MDEL database.
3.5.2. STERILISATION
No sterilization is required for the standard use of the equipment.
3.6. TRANSPORT AND STORAGE
During the transport and the storage it is necessary to respect the conditions indicated below.
Transport and storage temperature:
from -20° to +70° (Celsius)
Humidity conditions for transport and storage:
min 10%, max 85% (non-condensing)
Pressure:
710 –1060 hPa
Do not expose to acids, salts, rain.
EN
USER MANUAL
19
3.7. DEVICE DISPOSAL
3.7.1. INFORMATION FOR DEVICE OWNER
This symbol on the device indicates that it must not be disposed of together with other urban waste but it
is necessary to collect it separately.
The separate collection of this equipment is organised and managed by the manufacturer. When it is
necessary to dispose of this equipment, contact the manufacturer and follow the system that the
manufacturer has adopted to allow the equipment separate collection.
The separate collection and recycling of the equipment to be scrapped, contribute to the preservation of
the natural resources and ensure that such equipment is scrapped in respect of the environment and of
the health.
Illegal equipment disposal carries fines according to the local and regional laws.
To dispose of computers and other peripheral devices, it is necessary to refer to the attached instructions provided by
the manufacturer of the same devices.
3.7.2. INFORMATION FOR COLLECTION / DISPOSAL / RECOVERY FACILITIES
Separate the X-ray source, the electronic and mechanical parts, the plastic covers and the computer with the
peripheral devices.
The X-ray source contains oil that must be discharged to be disposed of and/or recovered.
The plastic parts must be disposed of with approved methods.
For all other parts for which the manufacturer does not provide specific information, refer to the national and local
laws and the guidelines on hygiene, safety at work and environmental protection.

20
USER MANUAL
4. STARTING PROCEDURES
This chapter provides an introduction to the NewTom VGi evo device, the switching on/off procedures and the control
devices located on the scanner.
4.1. INTRODUCTION TO THE SYSTEM
4.1.1. INTENDED USE
The device NewTom VGi evo is a cone beam computed tomography X-ray system. It is intended for diagnostic use
obtaining geometric information and radiologic density from two-dimensional and three-dimensional images of
anatomic particulars and objects in the examined area.
4.1.2. INDICATIONS FOR USE
The NewTom VGi evo is a computerised tomographic system using the cone-beam technology which acquires a
sequence of images of the head, including ear, nose and throat (ORL), of dental and maxillofacial unit, teeth,
mandible and jaw, temporomandibular joint (TMJ), other areas of the human cranium and neck with sections of the
cervical rachis for diagnostic use.
The device carries out such operations reconstructing a 3D matrix of the examined volume and producing two-
dimensional views of the volume (tomographic sections, panoramic and cephalometric projections) and then displays
two- or three-dimensional images.
The device is managed and used by doctors, dentists, radiologists and other legally qualified
professionals.
Its use is intended on the whole patient population for which radiodiagnostic analyses are usually carried out,
according to intended use, instructions for use, medical practice and local regulations.
It can also be used for X-ray analyses on implants, surgical templates and other diagnosis auxiliary tools.
Its use is intended in emergency situations, like first aid situations, only if a non-operation does not pose a hazard for
the patient (e.g. if the centre is equipped with other equivalent equipment or a department of radiology).
WARNING:
The NewTom VGi evo is able to produce panoramic reconstructions from CBCT
acquisitions. This may reduce the dose if both CBCT and panoramic images are needed.
However, if the system is used to simulate a panoramic
image when a CBCT acquisition is not necessary, the patient could be exposed to an
excessive dose of radiations.
WARNING:
The federal code limits the sale of this device only by or if prescribed by a doctor
authorised by the law of the State in which he uses or prescribes the use of X-ray imaging
systems 21CFR801.109 (b)
WARNING:
The imaging Cone Beam must not be used for routine (or "screening") examinations.
Other diagnostic tools must be taken into consideration. The imaging examinations must
be justified for each patient in order to prove that they provide more benefits than risks.
WARNING:
Where it is likely that an evaluation of soft tissues will be required as part of the patient X-
ray evaluation , the appropriate imaging should follow the “Diagnostic Imaging Referral
Guidelines of the Canadian Association of Radiologists”, instead of using the cone-beam
technology.
This manual suits for next models
1
Table of contents
Other NewTom Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Supersonic
Supersonic AixplorerUltimate user guide

BARDOMED
BARDOMED Care Pump EXPERT8 user manual
SamanTree Medical
SamanTree Medical Histolog Scanner v2 Instructions for use
Ivy Biomedical Systems
Ivy Biomedical Systems 3000T Operation manual
Ultrabreathe
Ultrabreathe Ultrabreathe User instructions

immedia
immedia 4WayGlide mini Instructions for use