7
II. How to Use Bello 2 Device
1. Precautionary Steps
Bello mobile application is only compatible with bello 1 and bello 2 devices.
1.1 Precautionary steps before operation
•Before using, please make sure to become familiar with the operating methods set forth in the user manual.
•Ensure that all product components are included in the product package.
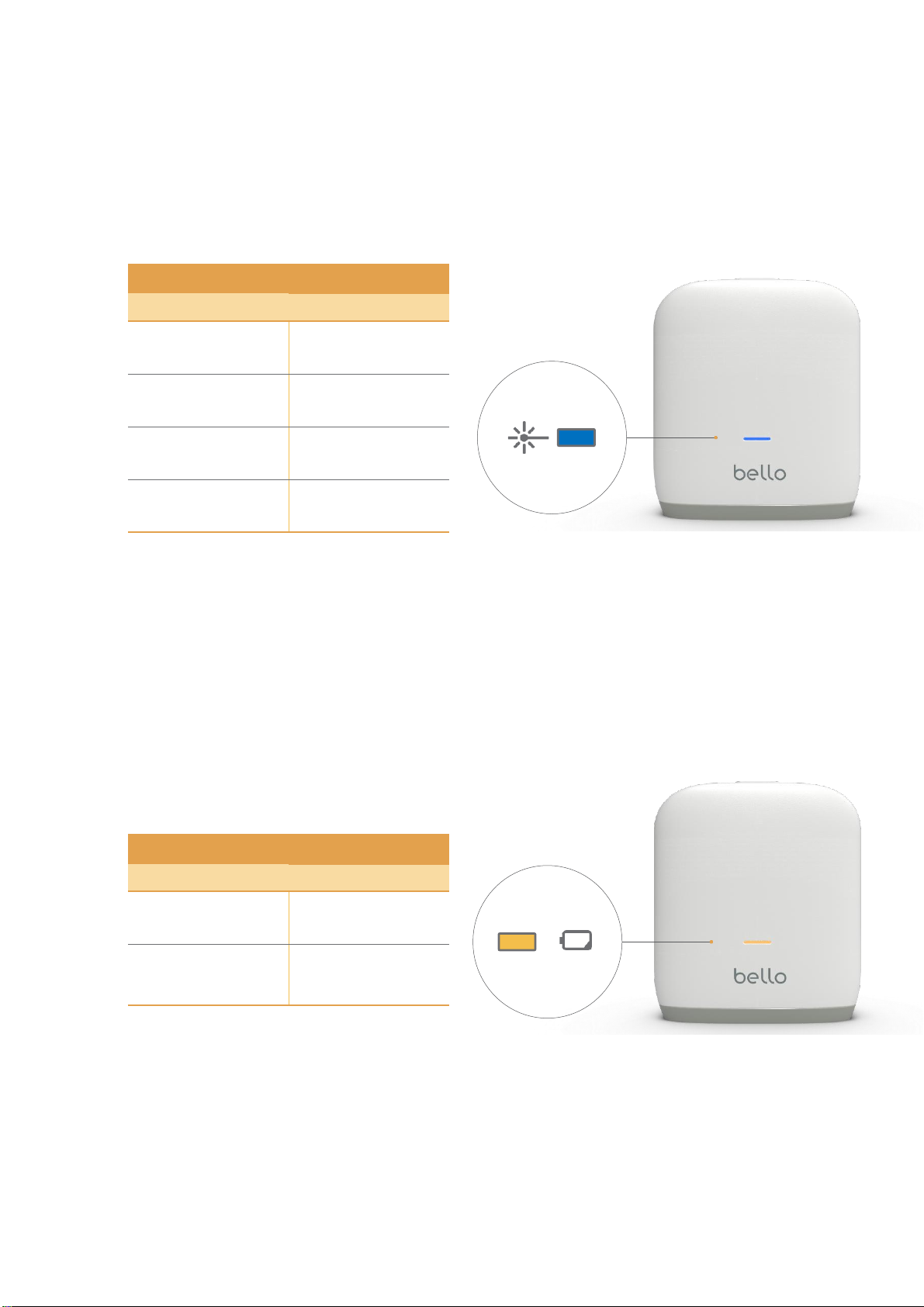
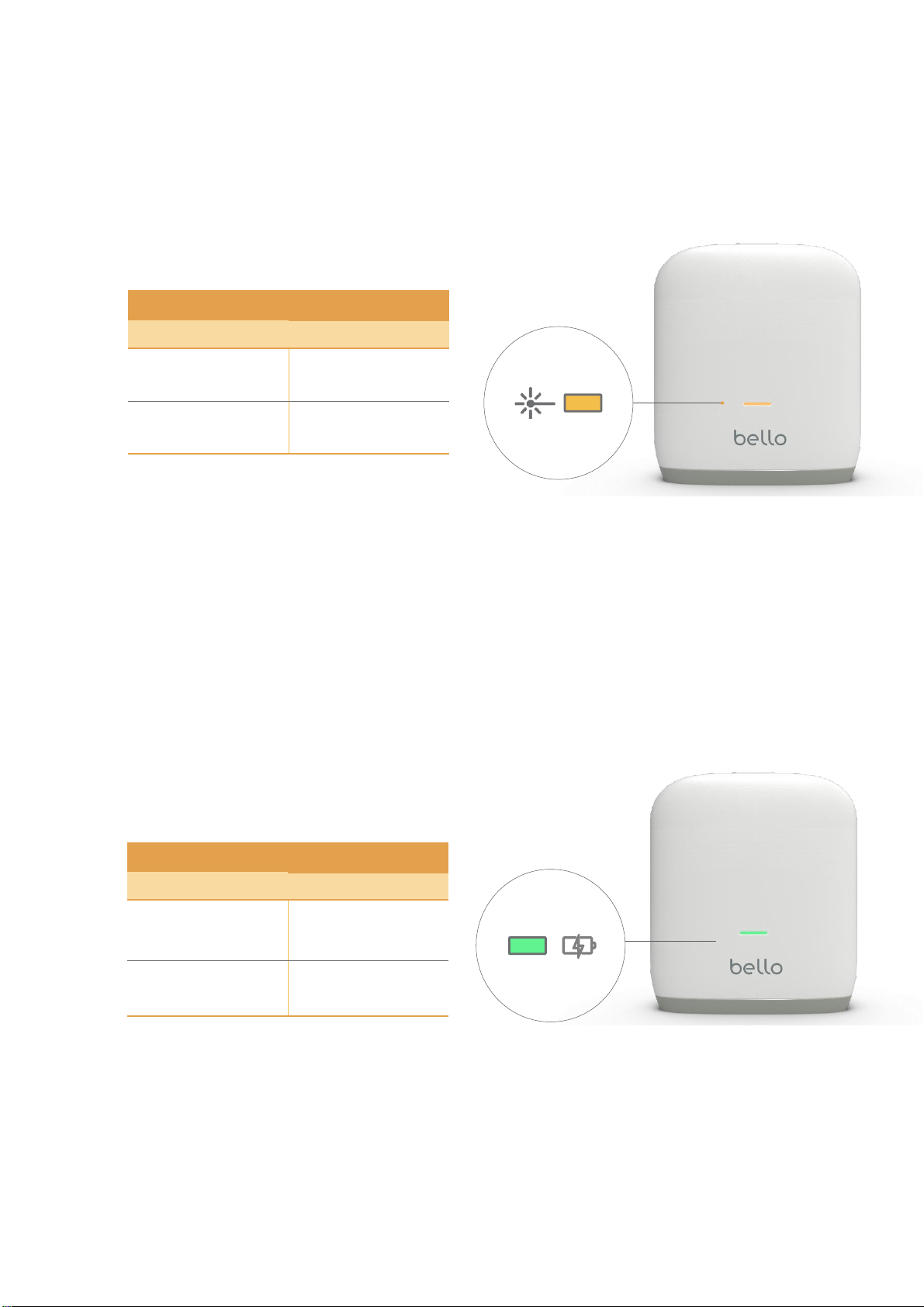
•Check the charging light and use the product after the device is charged sufficiently.
•If the charging cable is damaged or exposed to water, do not connect it to the product as it may cause
technical problems.
•Please use the following specifications regarding the adaptor :
(Direct current 5V, current 1A or more can be used)
•Please download the bello app from the iOS/Android app store and activate Bluetooth pairing mode on bello 2.
•Please ensure that your smartphone’s software version is higher than iOS 13.0 or Android 6.0.
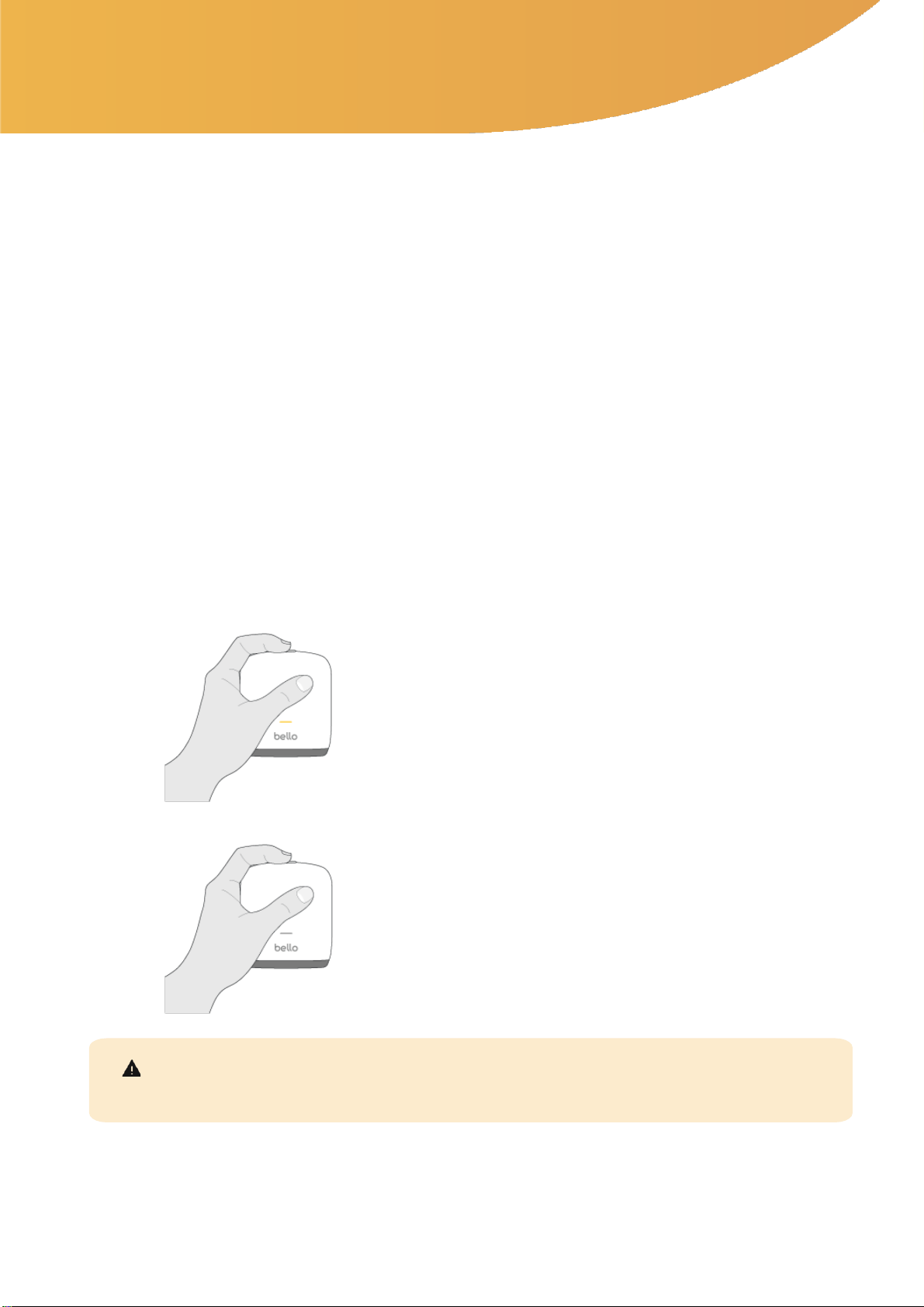
1.2 Turning the bello 2 device On / Off
•In order to turn the device off, press and hold the same
top button for more than 3 seconds.
Caution •Before scanning your body fat and belly fat, please ensure that your body hair and abdomen hair have been
removed and that your skinsurfaceis not wet or moistfor moreaccurate scanning results.
•In order to turn the device on, press and hold the top
button for 2 seconds.
•You will hear a “beep” sound and the device will stay on
for approximately 3 minutes.
•The device will turn off automatically if not used.
Power On
Power Off
•Regardless of whether the device is turned on or off, the
device is automatically turned on when the charging
cable is connected.