Produktserie GFS Ultimate (Deutsche)
1. Indikationen:
Die Produkte GFS Ultimate sind indiziert für den Einsatz zur Fixierung von Bändern und Sehnen bei
Patienten mit Reparaturbedarf für Bänder und Sehnen.
2. Kontraindikationen:
A. Aktive Infektionen aller Art.
B. Einschränkungen der Blutversorgung oder sonstige die Heilung verzögernde systemische
Zustände.
C. Eine etwaige vermutete Fremdkörperempfindlichkeit muss analysiert werden. Entsprechende
Vorsichtsmaßnahmen sind einzuhalten.
D. Unzureichende Qualität oder Quantität des Knochens.
E. Mangelnde Fähigkeit oder Bereitschaft des Patienten, die postoperativen Anweisungen des
Chirurgen einzuhalten.
F. Situationen, in denen der Benutzer außerstande ist, die Anwendungsvorschriften zu befolgen,
oder wenn das Produkt nicht bestimmungsgemäß verwendet wird.
3. Nebenwirkungen:
A. Tiefergehende als auch oberflächliche Infektionen.
B. Allergien und sonstige Reaktionen auf die Produktmaterialien.
C. Risiken bedingt durch die Anästhesie.
4. Warnhinweise:
A. Dieses Produkt ist zur Verwendung durch einen Arzt oder dessen Anordnung bestimmt.
B. Die mit diesem Produkt durchgeführte Fixierung ist bis zur vollständigen Heilung beizubehalten.
Bei Nichteinhaltung der vom Chirurgen angeordneten postoperativen Maßnahmen besteht die
Gefahr, dass das Produkt versagt und die Ergebnisse beeinträchtigt werden.
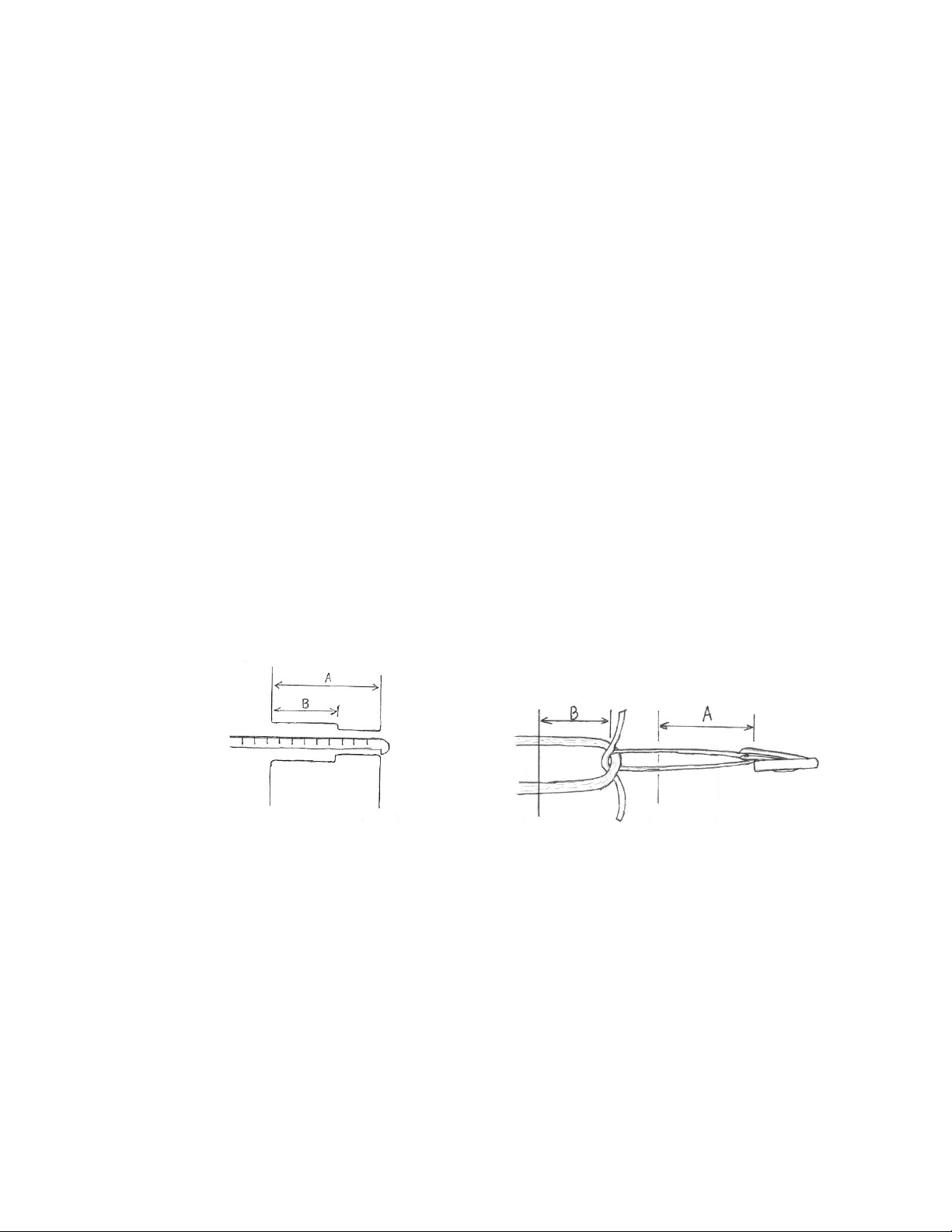
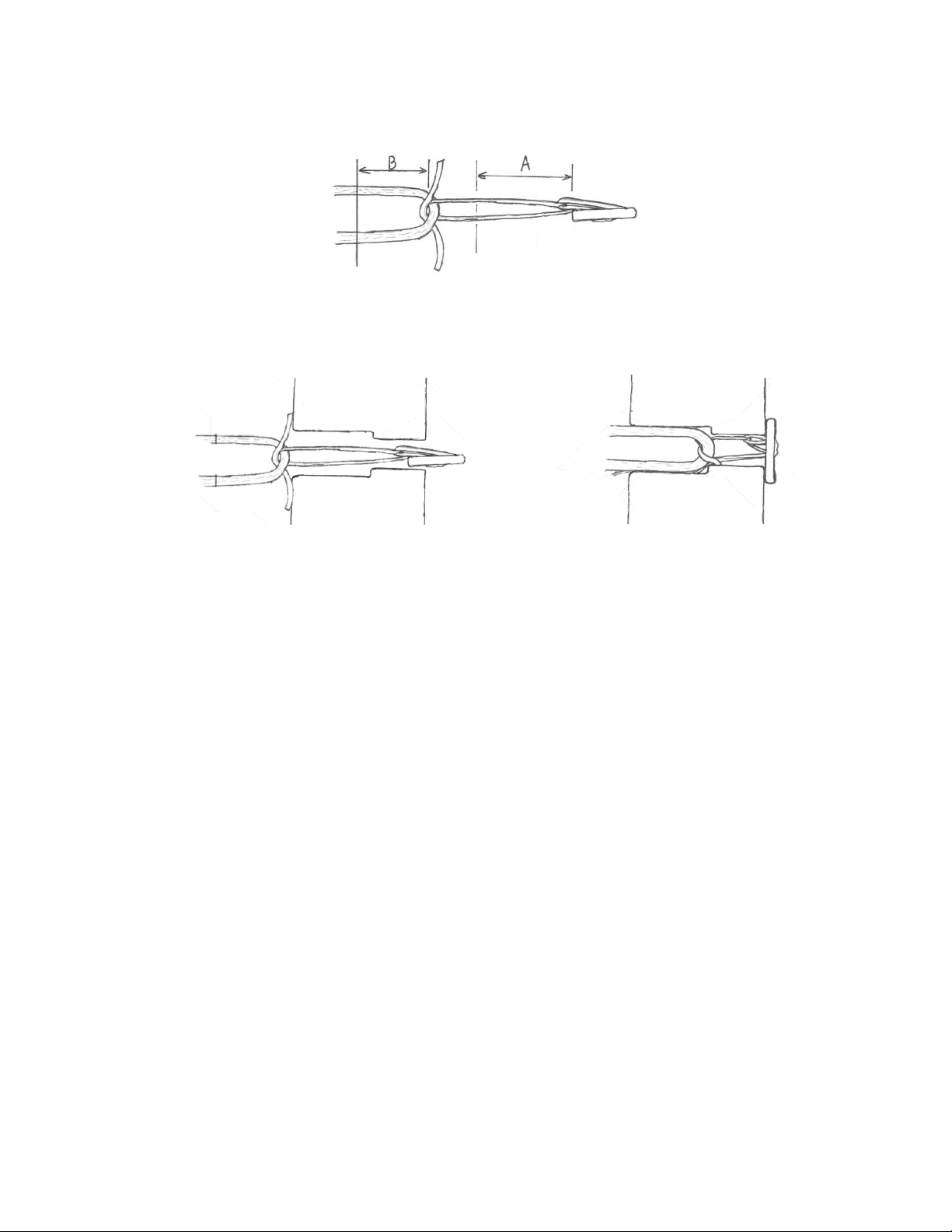
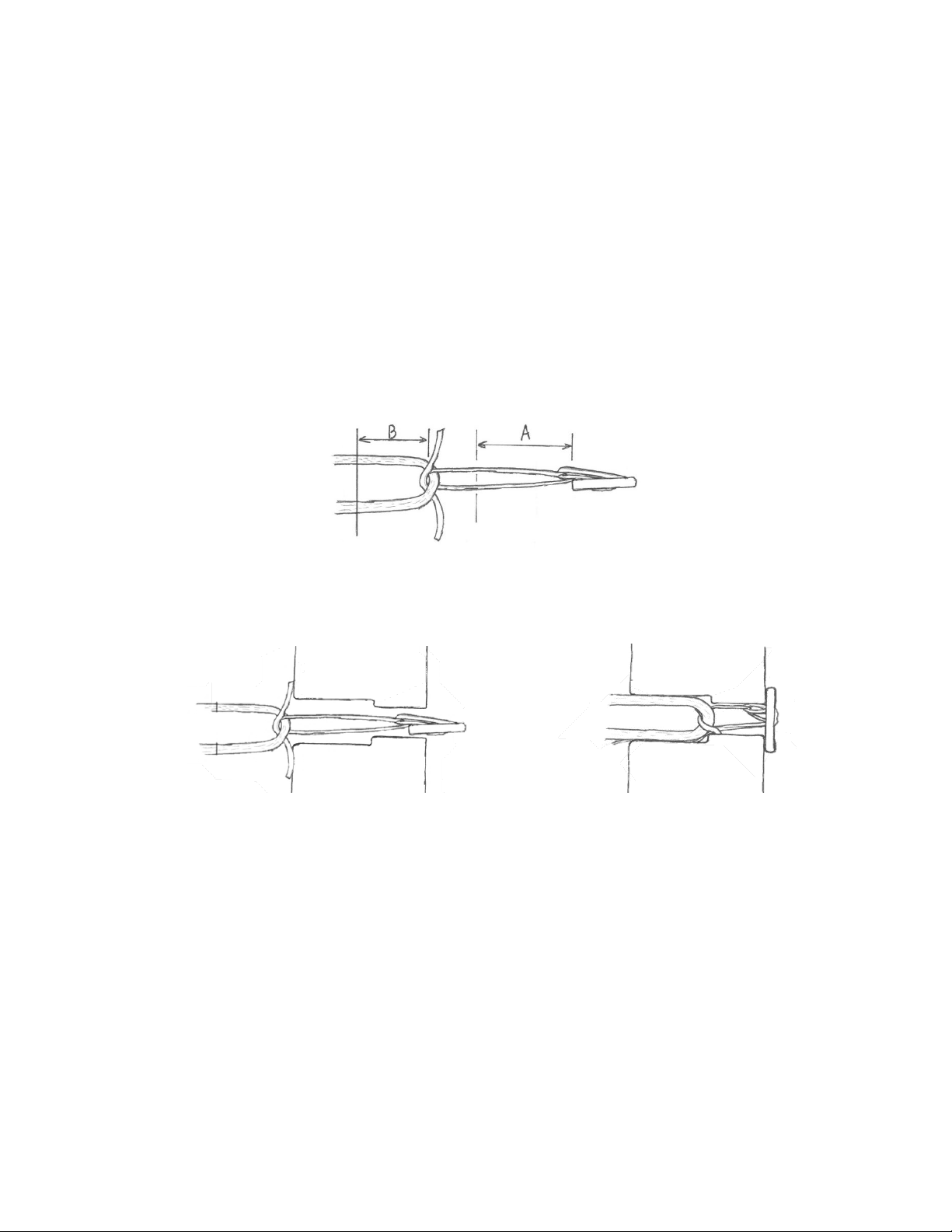
C. Die Größe des Implantats ist mit Sorgfalt auszuwählen. Zu berücksichtigen sind dabei die Qualität
des Knochens, auf dem das Implantat liegt, und die gewünschte Länge des in den Tunnel
einzubringenden Implantats.
D. Bei einer etwaigen Entscheidung, das Produkt zu entfernen, ist das potenzielle Risiko eines
zweiten chirurgischen Eingriffs abzuwägen. Nach dem Entfernen ist ein ordnungsgemäßer
postoperativer Managementplan zu erstellen.
E. Um ein gutes chirurgisches Ergebnis zu erzielen, sind präoperative Planung und Beurteilung, die
ordnungsgemäße chirurgische Vorgehensweise und Technik sowie die Verträglichkeit des
Implantats, einschließlich seiner Instrumente und Einschränkungen notwendig.
F. Das Produkt, einschließlich aller Inhaltskomponenten der sterilen Verpackung, darf grundsätzlich
nicht wiederverwendet werden. Wiederverwendung oder Resterilisation können zu
Veränderungen der Materialcharakteristik, wie Deformationen und Materialverschlechterung,
führen und die Leistung des Produkts beeinträchtigen. Überdies kann die Wiederaufbereitung
von Einwegprodukten Kreuzkontaminationen und damit Infektionen seitens des Patienten
verursachen.
G. Dieses Produkt, einschließlich aller Inhaltskomponenten der sterilen Verpackung, darf
grundsätzlich nicht resterilisiert werden.
H. Zum Implantieren dieses Produkts sind die richtigen Instrumente zu verwenden.