http://www.parker.com/ppf
Page 7
IV. BACKGROUND
In 1977, Dr. R.E. McCaman and associates
provided a complete description (1) of a pressure
ejection system that utilized a high speed valve.
This valve continues to be the heart of the pressure
system offering very precise control of ejection
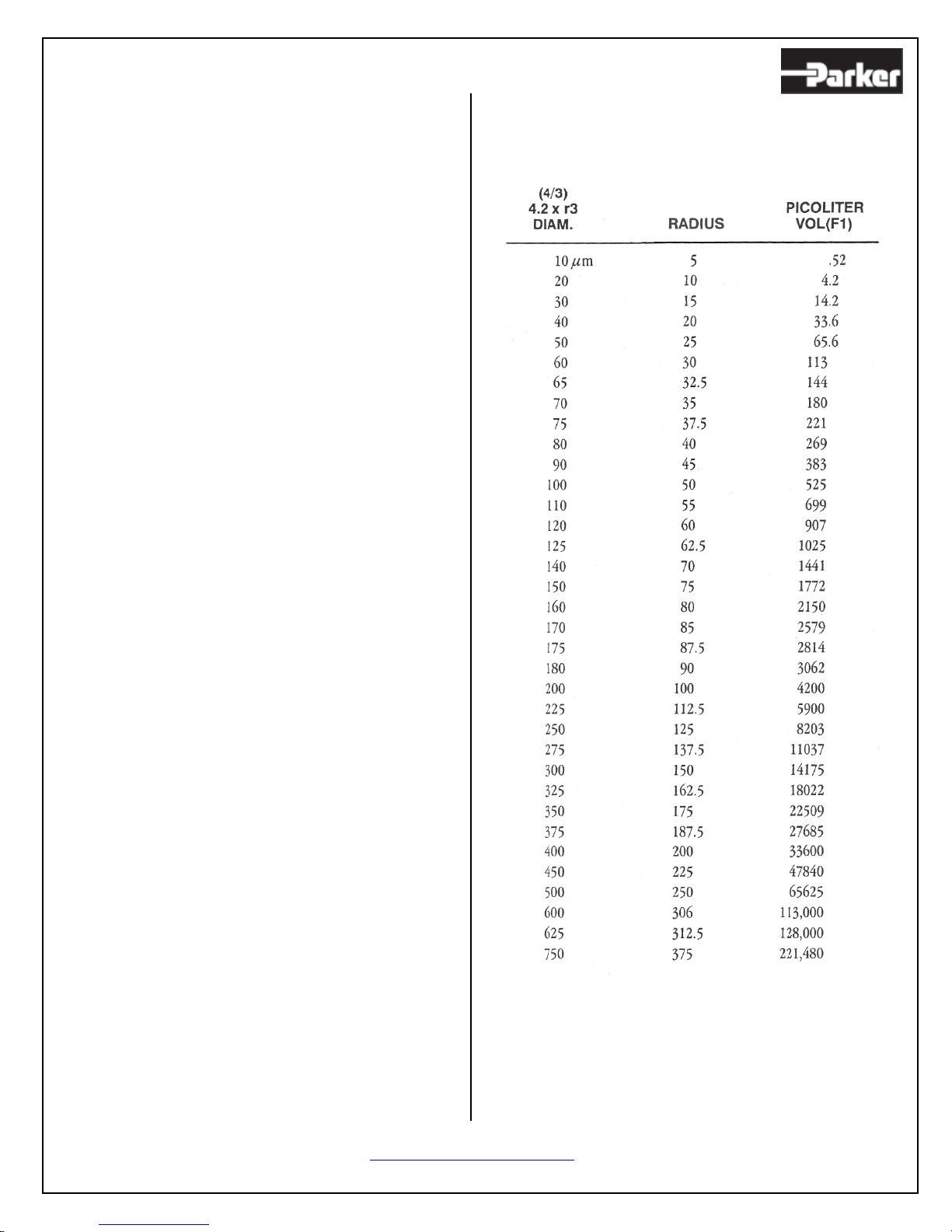
volumes (in the picoliter range) and ejection times
(in the millisecond range).
Furthermore, these investigators described a series
of holders that permitted ejection through
micropipettes with sufficiently small tips that could
be used for simultaneous intracellular recordings
during ejections.
These systems have been used for intracellular as
well as extracellular ejections. In listing advantages
of the pressure system, these investigators
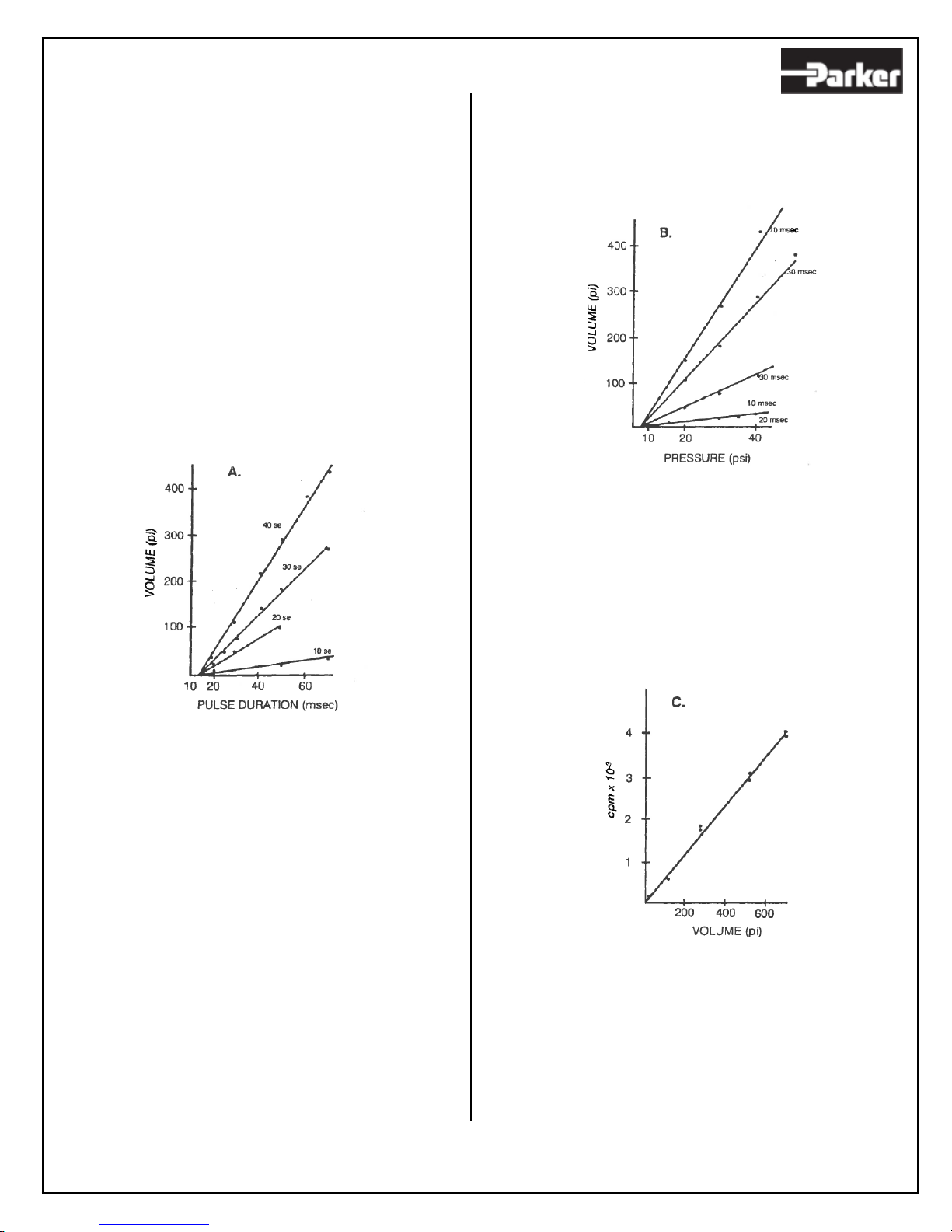
emphasize that the linear relationship between
ejection volume and either duration of the pulse or
of the applied pressure permits a rapid, convenient
and reliable calibration of each pipette (1, 3), unlike
that for electrophoretic techniques (7-9).
Pressure ejection seems an ideal approach to
delivering uncharged substances such as peptides
(4, 6), steroids (4), and enzymes (2,5). The
solutions used for pressure ejections are usually
several orders of magnitude more dilute than those
used for electrophoretic ejection (1, 3), thus
avoiding receptor desensitization commonly
experienced with iontophoresis. The fact that the
ejection efficiency of the pneumatic systems is not
influenced by solute concentration nor by net
charge, makes them ideal for intracellular injections
of radiolabeled or tracer substances (13-15).
Thus, pressure systems have been used for
intracellular injection of radiolabeled precursors or
neurotransmitters (10, 11) and [H3] –sugars as
precursors of glycoproteins (12) in order to study
neuron-specific transmitter biosynthesis, axonal
transport and cellular topography. The reproducible
and quantifiable ejections obtained with pressure
systems make them ideal for neuropharmacological
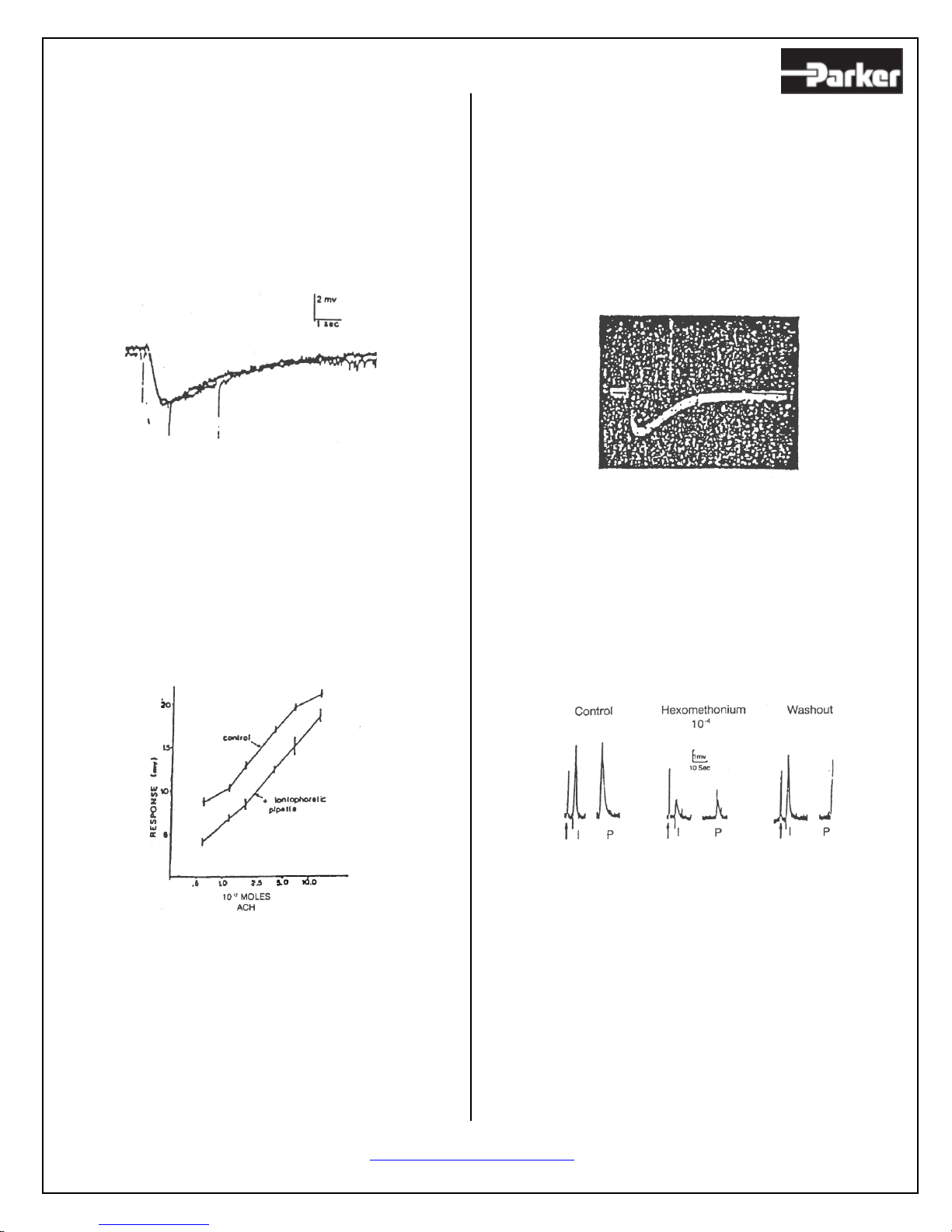
studies of agonist and drug interactions with
membrane receptors (1, 3, 4).
As you find additional uses for your Picospritzer,
please send us a reprint for addition to our reference
section so that others may benefit from your
experience.
N.B.; H3=radioactivity (tritium) label substance.
REFERENCES
References describing the use and unique advantage
of pressure systems in several types of
experimentation in the field of neurobiology, cell
biology, and biophysics are:
1. McCaman, R.E., Mc Kenna, D.G. and Ono, J.K.
“A pressure system for intracellular and extracellular
ejections of picoliter volumes.” Brian Research
136:141 (1977).
2. Sakaki, M., Sakai, H. and Woody, C.D.
“Intracellular staining of cortical neurons by pressure
micro-injection of horseradish peroxidase and
recovery by core biopsy.” Exp. Neurol. 58:138
(1978)
3. Sakai, M., Swartz, B.E. and Woody, C.D.
“Controlled micro release of pharmacological agents:
measurements of volume ejected in vitro through fine
tipped glass microelectrodes by pressure.”
Neuropharmacol. 18:209 (1979)
4. Dufy, B., Vincent J-D. Fluery, H., Pasquier, P.,
Gourdji, D., and Tixler- Vidal, A. “Membrane effects
of thyrotropin-releasing hormone and estrogen shown
by intracellular recording from pituitary cells.”
Science. 204.509 (1979)
5. Tauc, L., Hoffman, A., Tsuji, S., Hinzen, D. and
Faille, L. “Transmission abolished in a cholinergic
synapse after injection of acetylcholinesterase into
the presynaptic neuron.” Nature, 250, 496 (1974)
6. Chang, J.J., Gelperin, A., and Johnson, F.H.
“Intracellulary injected aequorin detects
transmembrane calcium flux during action potentials
in an identified neuron from the terrestrial slug.”
Brain Research 77:431 (1974)