CONTENTS
1. Introduction.................................................................................................................................... 1
2. Service manual terms and safety symbols.................................................................................... 1
3. Warnings ....................................................................................................................................... 1
4. Technical specifications ................................................................................................................ 4
4.1. Classification....................................................................................................................... 4
4.2. Electrical requirements ....................................................................................................... 4
4.3. Compliance......................................................................................................................... 4
4.4. Dimensions ......................................................................................................................... 5
4.5. Environmental conditions.................................................................................................... 5
4.6. Computer minimum requirements....................................................................................... 5
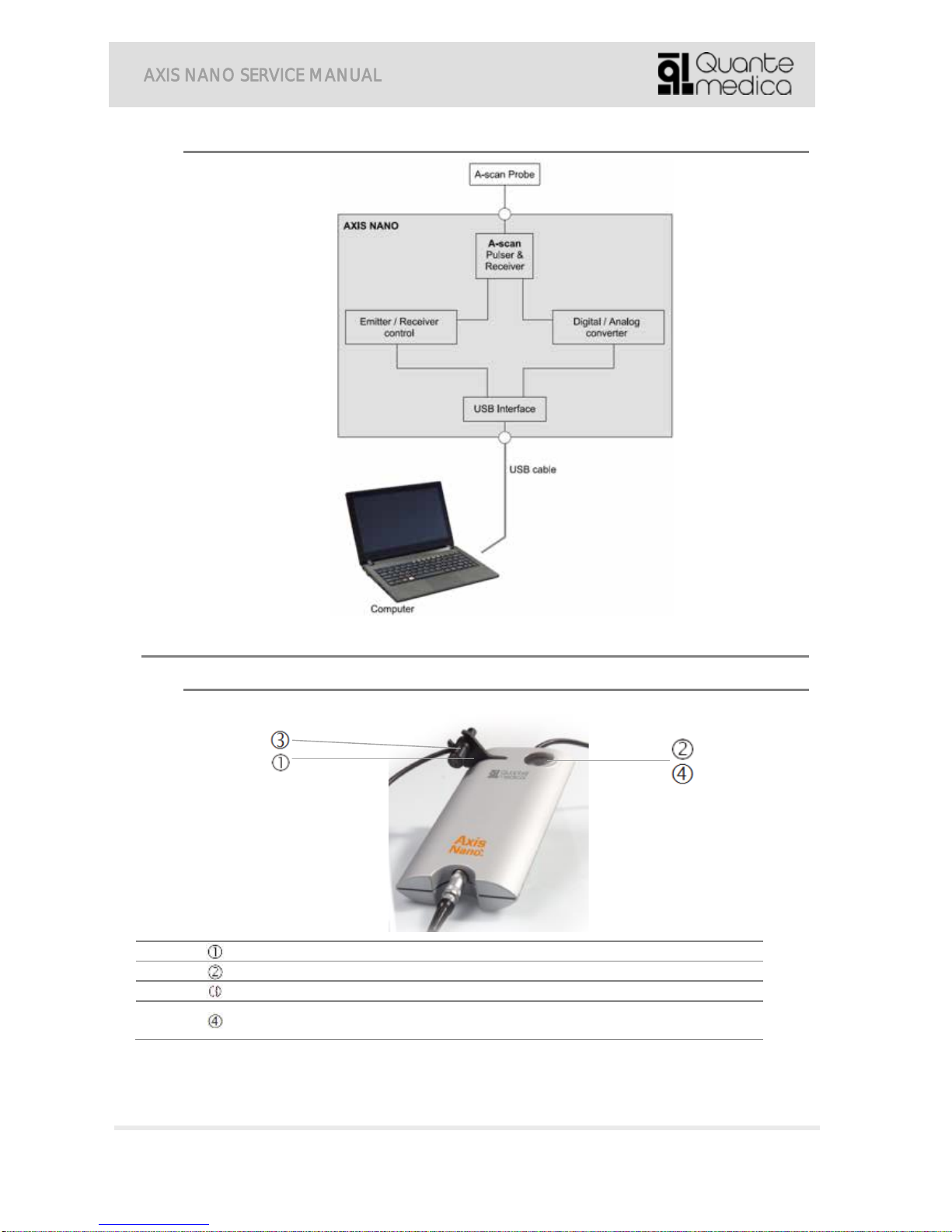
4.7. Block diagram ..................................................................................................................... 6
5. Accessories and spare parts......................................................................................................... 6
5.1. External view....................................................................................................................... 6
5.2. Spare parts.......................................................................................................................... 7
6. Markings........................................................................................................................................ 8
7. Disassembling............................................................................................................................... 9
7.1. Probe holder removal.......................................................................................................... 9
7.2. Cover removal..................................................................................................................... 9
7.3. Acquisition board............................................................................................................... 10
8. Location and connector pin description....................................................................................... 11
9. Database information and backup .............................................................................................. 12
9.1. Automatic backup (only available from version over 1.03)............................................... 12
9.2. Database information........................................................................................................ 12
9.3. Manual database backup and restoration......................................................................... 13
9.3.1. To manually make a database backup.............................................................. 13
9.3.2. To manually restore the database on the axis nano unit................................... 13
10. Software installation.................................................................................................................... 14
10.1. Software compatibilities .................................................................................................... 14
10.1.1. Software and operating system compatibilities................................................. 14
10.1.2. Software upgrade and compatibilities ............................................................... 14
10.1.3. Software and screen resolution compatibilities................................................. 14
10.1.4. Axis nano software behavior with UAC on or UAC off ...................................... 15
10.2. Installation warnings and cautions.................................................................................... 16
10.3. Upgrade procedure........................................................................................................... 16
10.3.1. Uninstalling previous software version.............................................................. 16
10.3.2. In case the axis nano software version 1.04 cannot be uninstalled.................. 17
10.4. Installation procedure........................................................................................................ 17
11. Hardware installation................................................................................................................... 20
12. USB driver installation................................................................................................................. 21
13. AXIS NANO software display & adustments............................................................................... 22
13.1. Navigation properties setup with windows®eight (8.1).................................................... 22
13.2. Adjust magnifier tool.......................................................................................................... 23
13.3. Suppressing the access to switch « user account »......................................................... 23
14. AXIS NANO setup....................................................................................................................... 23
15. Hidden screen ............................................................................................................................. 25
15.1. Accessing the hidden screen............................................................................................ 25
15.2. Hidden screen functions ................................................................................................... 26
a.“zero” field and “position of marker #1 in contact” ............................................................ 26
b.Retina slope test ............................................................................................................... 26
c. Biometry default mode...................................................................................................... 27
d.Field “d2/low dyn”.............................................................................................................. 27
e.Keycode ............................................................................................................................ 27
f. Main icons displayed by default........................................................................................ 27
16. Probes calibration........................................................................................................................ 28
16.1. Calibration check............................................................................................................... 28
16.2. Calibration adjustment ...................................................................................................... 30
16.3. Using the hidden screen to reset the “ position of marker #1 in contact ” to zero ............ 32
17. AXIS NANO – no top most mode................................................................................................ 33