Chapter 1 Safety Notices
4Model QS-550 - Operator’s Manual
REGULATORY COMPLIANCE
The Model QS-550 Tubestand is not a CDRH certifiable product. However, it must be
compatible and operate in conjunction with other components in the x-ray system so
that the x-ray system performs in compliance with H.E.W. Performance Standards.
This product has been factory tested to assure its required performance in an x-ray
system.
Those responsible for the planning of x-ray equipment installations must be thor-
oughly familiar and comply completely with NCRP Report No. 49, "Structural Shielding
Design and Evaluation for Medical Use of X-Rays and Gamma Rays of Energies up to
10 MeV", as revised or replaced in the future. Those authorized to operate, test, par-
ticipate in or supervise the operation of the equipment must be thoroughly familiar
and comply completely with the currently established safe exposure factors and pro-
cedures described in publications such as Subchapter J of Title 21 of the Code of Fed-
eral Regulations, "Diagnostic X-Ray Systems and Their Major Components," and NCRP
Report No. 102, “Medical X-Ray, Electron Beam and Gamma Ray Protection for Ener-
gies Up to 50 MeV—Equipment Design and Use” as revised or replaced in the future.
Scheduled maintenance is essential to the assurance of continued integrity of this
equipment with respect to regulatory compliance. The continuance of certified perfor-
mance to the regulatory standard is incumbent upon the user's diligent conformance
to recommended maintenance instructions.
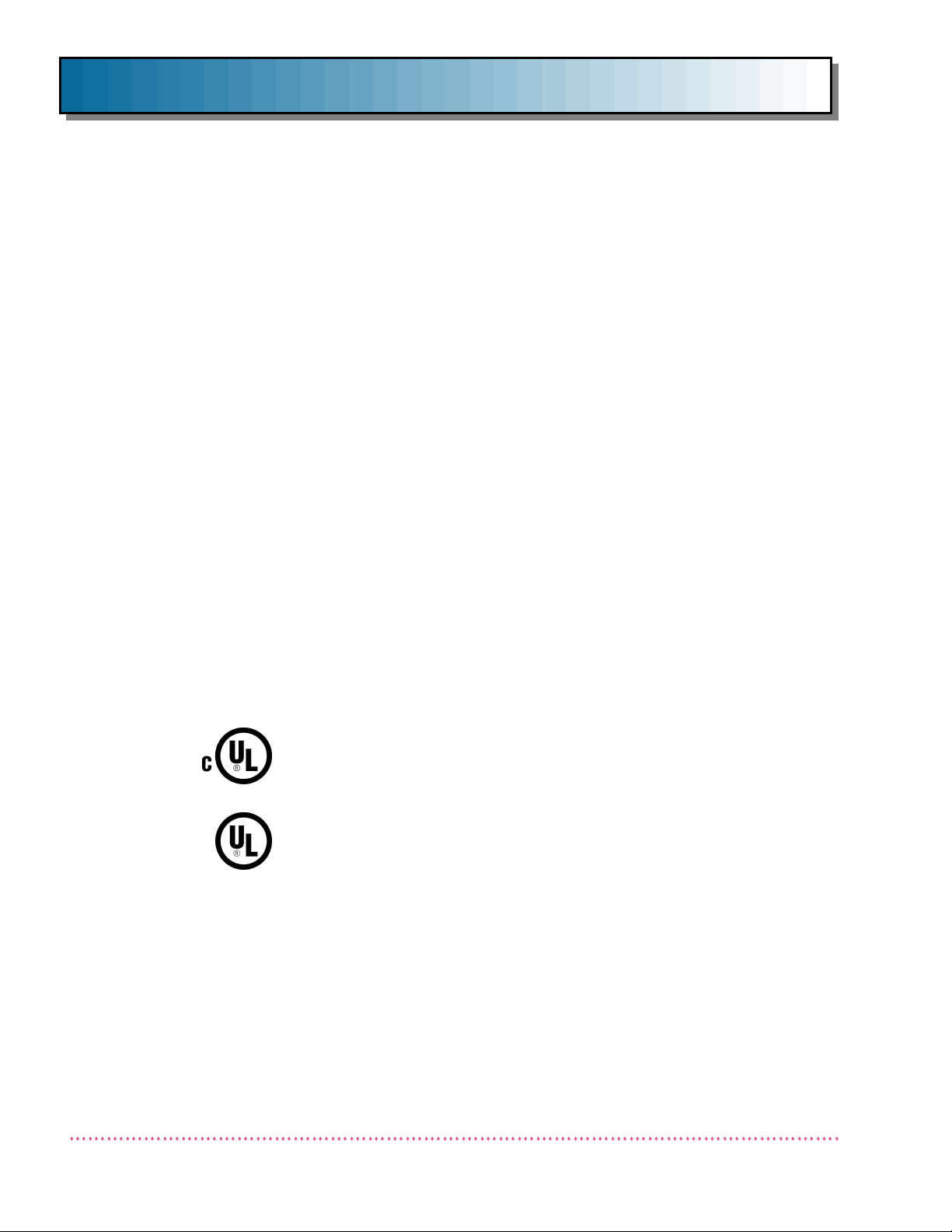
This product has been classified as Class I, Type B by Underwriters Laboratories, Inc.
Equipment not suitable for use in the presence of a flammable anesthetic mixture of
air with oxygen or with nitrous oxide.
MEDICAL ELECTRICAL EQUIPMENT
WITH RESPECT TO ELECTRIC SHOCK, FIRE,
MECHANICAL HAZARDS ONLY
IN ACCORDANCE WITH CAN/CSA C22.2 NO. 601.1
98YA
MEDICAL ELECTRICAL EQUIPMENT
WITH RESPECT TO ELECTRIC SHOCK, FIRE,
MECHANICAL HAZARDS ONLY
IN ACCORDANCE WITH UL 2601-1
98YA