Siare Morpheus E User manual

Anaesthesia Unit
Morpheus E
User's Manual


MORPHEUS III
GENERAL INFORMATION
The information contained in this manual are the exclusive property of SIARE
ENGINEERING INTERNATIONAL GROUP s.r.l. and may not be reproduced in
any way without authorisation. SIARE ENGINEERING INTERNATIONAL GROUP
s.r.l. reserves the
right to modify or replace this manual at any time without prior
notice.
It is however recommended that you make sure you have the most recent version
of the manual. In the event of doubt, contact SIARE ENGINEERING
INTERNATIONAL GROUP s.r.l. (see the addr
ess on page IX). The information
contained herein can be considered correct, but do not exclude
professional
knowledge by the user of the equipment.
The operation and maintenance must be entrusted to qualified technical personnel
only. The responsibility
of SIARE ENGINEERING INTERNATIONAL GROUP s.r.l.
as regards the anaesthesia unit and its use is limited to what is indicated in the
guarantee supplied with the equipment.
The contents of this manual do not in any way limit the right of SIARE
ENGINEERING IN
TERNATIONAL GROUP s.r.l. to revise, change or modify
without prior notice the equipment (including the relative software) described
herein.
Unless otherwise specifically agreed in writing, SIARE ENGINEERING
INTERNATIONAL GROUP s.r.l. is not obliged to sup
ply such revisions, changes
or modifications to the owner or user of the equipment (including the relative
software) described herein.
The information contained in this manual refers to the versions of MORPHEUS
anaesthesia unit produced or updated after May 2019.
It is possible that some
information may not apply to previous versions. Contact SIARE ENGINEERING
INTERNATIONAL GROUP s.r.l. if you have any doubts.
User’s Manual, version DU3300105
Revision 5 - 02.05.2019.

IV User’s Manual, version DU3300105
Observations
SIARE Engin
eering International Group s.r.l. wishes to thank you for purchasing
one of its products.
Any comment on the accuracy and usefulness of this User’s Manual would be
very helpful in allowing us to guarantee current and future users of the high quality
level
of our manuals. We would be grateful if you would send us your comments
(see address at page IX).
The SIARE trademark is used throughout this manual as an abbreviation for the
manufacturer: SIARE Engineering International Group s.r.l.
Directive 93/42 EEC
Definitions
Three symbols are used in this User’s Manual to indicate particularly important
information.
WARNING!
This indicates a condition of danger for the patient or for the
operator.
CAUTION
This indicates the possibility of danger to the equipment.
NOTE
This indicates information worthy of note, making the operation
of the of MORPHEUS anaesthesia unit more efficient or
practical.

MORPHEUS V
Warnings, cautions and notes
You are advised to carefully read the information give
n alongside the three
symbols shown on the previous page, since it contains considerations on
the
safety, the special requirements for the use MORPHEUS anaesthesia unit and
the relative safety regulations.
•In order to understand how the MORPHEUS anaesthesi
a unit works and how
to use it correctly to ensure patient and user safety, the recommendations
and instructions contained in this manual must be read with care and
understood.
•
The anaesthesia unit must only be used for the purposes specified herein
and th
e safety of the equipment is therefore only guaranteed if it is used in
accordance with the instructions given in this User’s Manual.
•
The materials used were carefully selected during the design stage after
specific checks, tests and comparative trials: th
ese materials are also
constantly inspected during the production cycle to achieve the best results in
terms of reliability and safety for the patient and the operator. Any part of
circuit must therefore only be replaced with original spare parts supplied
or
checked by SIARE.
•
The anaesthesia unit must only be used by qualified personnel and only in
equipped and dedicated rooms, according to the regulations in force in the
country where the equipment is installed.
•To ensure correct technical assistance and a
void possible physical damage
to the patient, the maintenance schedule foreseen in this manual must be
respected; qualified personnel must only carry out maintenance of the
anaesthesia unit or authorised modifications to the equipment. The user of
this pro
duct is solely responsible for any operating defect caused by improper
use or interventions carried out by third parties other than specialised SIARE
personnel.
•
For any repairs to anaesthesia unit (due to malfunctioning, defects or
failures), the user must
contact SIARE or the authorised local Technical
Service Centre; it is advisable to specify the data on the identification label
(model, serial number, ……) when requesting intervention.
•SIARE recommends establishing a maintenance and service contract wit
h
SIARE or the local authorised service dealer in order to guarantee the
scheduled maintenance required to operate the anaesthesia unit in a safe
and correct manner.
•To prevent the risk of fire, keep the anaesthesia unit
and/or the oxygen tubes
of the equipm
ent away from matches, lit cigarettes and inflammable material,
such as anaesthetic gases and/or sources of heat.

VI User’s Manual, version DU3300105
•
Do not connect the anaesthesia unit to the patient by flexible connectors, and
antistatic or conductive tubes to prevent patient burnings
during the use of
high frequency surgical equipment, specially dangerous with antistatic tubes.
The use of flexible connectors, antistatic or conductive tube is never
permitted with MORPHEUS anaesthesia unit.
•Do not use worn and consumed tubes or tubes co
ntaminated by flammable
substances like grease or oil to deliver oxygen; (fabrics, oil and other fuels
can easy ignite and they intensively burn in air with high concentration of
oxygen.
•In the event of fire or an unpleasant smell (e.g. a smell of burning)
, the
anaesthesia unit
should immediately be disconnected from the electrical
power supply and from the battery (if fitted).
•
When coming into contact with any component of the anaesthesia unit, the
hospital procedures for the handling of infected material
should always be
respected.
•
SIARE is aware that cleaning, sterilisation and disinfection procedures vary
considerably from one health structure to another. SIARE cannot be held
responsible for the efficacy of the cleaning and sterilisation procedures, nor
for the other procedures carried out while the patient is being treated. As
regards cleaning, sterilisation and disinfection of the product components, it is
therefore recommended that the regulations currently in force in the country
where the equipment is installed be taken into consideration.
•
The MORPHEUS anaesthesia unit was not designed as a total monitoring
device: some conditions of danger for the patients treated with vital support
equipment will not trigger any alarm.
•Before using the anaesthesia unit
or any connected component, carefully
check that the equipment is functioning correctly; when needed, the auto-
diagnostic test must be performed as described in the present User’s Manual.
•Do not use pointed instruments, such as pencils, screwdrivers o
r the like to
make selections or settings as they could damage the surface of
the LCD
panel.
•
Check the anaesthesia unit periodically as described in the relative
“Maintenance” chapter and do not use it if it is faulty or malfunctioning.
Replace any broken,
missing, obviously worn, deformed or contaminated
parts immediately, with spare parts supplied by SIARE.
•
Do not connect external devices NOT manufactured or NOT authorized by
SIARE to the equipment (example
: scavenging systems, patient simulators,
etc…..
), and not described in the present user’s manual: in case of need
contact SIARE.

MORPHEUS VII
•
The correct functioning of the anaesthesia unit can be impaired if original
SIARE spare parts and accessories are not used; the use of other
accessories is however allo
wed only if formally authorised by SIARE
in accordance with current safety regulations.
•
SIARE assumes all foreseen legal liability if the anaesthesia unit is used and
periodically maintained according to the instructions contained in this manual:
the Techn
ical Assistance Report, drawn up and signed by the authorised
SIARE technician, is proof of the completion of the scheduled maintenance.
•
Notwithstanding the MORPHEUS anaesthesia unit is equipped with a safety
valve which allows to the patient to breathe sp
ontaneously the ambient air
even in case of gas supply failure, the auxiliary ventilation system must be
always promptly available; such a component is part of SIARE
ENGINEERING INTERNATIONAL GROUP s.r.l. products range.

VIII User’s Manual, version DU3300105
WARNING !!
•The MORPHEUS is not approved for operation in places where
there is any risk of explosion.
•
Do not use the MORPHEUS in the presence of flammable
gases.
•
The MORPHEUS cannot be used in the presence of explosive
gases.
WARNING !!
•The MORPHEUS shall not be used in a hyperbaric chamber.
•The MORPHEUS shall not be used with nitric oxide.
•The MORPHEUS shall
not be used with helium or mixtures
with helium.
WARNING !!
•
Before starting the MORPHEUS use, you have to carry out the
preliminary checks.
•Qualified staff mu
st make the regulation of ventilation
parameters.
•Do not block the
gas intake port or emergency intake port
(valves group), thereby interfering with PATIENT ventilation.
WARNING !!
Before connecting the MORPHEUS to other electrical equipment
not de
scribed in this manual, a request for authorisation should be
sent to Siare.
WARNING !!
An auxiliary ventilation system is suggested for the patients for
which the anaesthesia unit represents a life support.
WARNING !!
Independent ventilation tools
should be available (i.e. manual
resuscitator bag equipped with face
mask) each time the
ANESTHESIA UNIT is in use.
MORPHEUS IX
SIARE declines all civil and penal responsibility in the
following cases:
•If the anaesthesia unit is used in conditions and f
or purposes
not stated or described in this manual.
•If the anaesthesia unit is used by non-qualified personnel.
•
If periodic maintenance as foreseen by this manual has not
been carried out correctly or has been skipped.
•If personnel not officially authorise
d by SIARE have performed
maintenance.
•If non-
original SIARE spare parts or components not checked
by SIARE have been used.
•
If the anaesthesia unit has been connected to equipment not
complying with the safety norms for the intended use.
•Direct or indirect
damage to persons or things caused by
unauthorised technical intervention or by improper use of the
anaesthesia unit not in accordance with the instructions
contained in the users and maintenance manual.
Year ofmanufacture
Check the identification data label of the MORPHEUS anaesthesia unit in the
relative chapter.
Shelf lifeofmedical device
The Directive 93/42EEC on medical devices foresees that the manufacturer
defines the shelf life of the device according to the intended purpose. The shelf life
foreseen by SIARE for the MORPHEUS anaesthesia unit is 10 years.
Manufacturer
SIARE Engineering International Group s.r.l.
Via Giulio Pastore, 18 - 40056
Località Crespellano, 40053 Valsamoggia (BO), ITALY
Tel.: +39 051 969802 - Fax: +39 051 969809
E-mail: mail@siare.it - web: www.siare.it

X User’s Manual, version DU3300105
Electromagnetic Compatibility
The MORPHEUS anaesthesia unit is designed to operate in the specified
electromagnetic environment (see warning below).
The customer or the user of MORPHEUS anaesthesia unit should ensure that it
is used in such an electromagnetic environment.
The MORPHEUS anaesthesia unit complies with the EN 60601-
1-2 regulations on Electromagnetic Compatibility of electro-
medical equipment. It is in any case highly recommended not to
use the anaesthesia unit adjacent to high-
powered equipment
or to units, which emit strong electro-
magnetic fields. Mobile
phones, cordless phones or other radio transmitters used in the
vicinity of the equipment could influen
ce its operation.
Whenever the anaesthesia unit should be necessarily used
nearby to such equipment, it will be required to supervise its
normal operation.
In general, as regards the regulations regarding
“electromagnetic emissions”, “electromagneti
c immunity”
and
“recommended separation distances between portable
and
mobile RF equipment and the device”, always refer to
what
is described in the MORPHEUS anaesthesia unit user’s
manual.
Requirements applicable to cables, transducers and other
accessories
that could affect compliance with the requirements
of 6.1 and 6.2

MORPHEUS XI
Table of contents
General Information .......................................................................................................III
Observations...................................................................................................................................IV
Definitions .......................................................................................................................................IV
Warnings, cautions and notes .........................................................................................................V
Year of manufacture .......................................................................................................................IX
Shelf life of medical device .............................................................................................................IX
Manufacturer...................................................................................................................................IX
Electromagnetic Compatibility .........................................................................................................X
Table of contents ............................................................................................................................XI
1 INTRODUCTION.....................................................................................................1-1
1.1 Foreseen use....................................................................................................................... 1-1
1.2 Versions............................................................................................................................... 1-2
1.3 Main technical characteristics.............................................................................................. 1-2
1.3.1 Trolley.....................................................................................................................................1-2
1.3.2 Valves group...........................................................................................................................1-3
1.3.3 Flowmeter box........................................................................................................................1-4
1.3.4 Lung ventilator - basic DISPLAY ............................................................................................1-4
1.3.5 Lung ventilator - ventilator with advanced 12” TFT display.....................................................1-4
1.3.6 Various on lung ventilator.......................................................................................................1-5
1.4 Correct operation................................................................................................................. 1-6
1.5 Applicable norms................................................................................................................. 1-7
2 DESCRIPTION........................................................................................................2-1
2.1 Introduction.......................................................................................................................... 2-2
2.2 Versions............................................................................................................................... 2-3
2.2.1 MORPHEUS LT......................................................................................................................2-3
2.2.2 MORPHEUS M.......................................................................................................................2-4
2.2.3 MORPHEUS E .......................................................................................................................2-5
2.3 Trolley.................................................................................................................................. 2-6
2.3.1 Support for vaporizers............................................................................................................2-6
2.3.2 Control panel for manual ventilation ( 9 )................................................................................2-7
2.4 Side view ............................................................................................................................. 2-9
2.4.1 Valves group ( 10 ) ...............................................................................................................2-10
2.4.2 Connections for scavenger ( 21 )..........................................................................................2-12
2.4.3 Electric power supply group ( 22 )........................................................................................2-12
2.5 Back view........................................................................................................................... 2-13
2.5.1 Gas supply group ( 23 )........................................................................................................2-14
2.5.2 Connectors for services ( 24 )...............................................................................................2-15
2.6 Product identification label................................................................................................ 2-16

XII User’s Manual, version DU3300105
3 VALVES GROUP MODULE....................................................................................3-1
3.1 Introduction.......................................................................................................................... 3-2
3.2 Valves group........................................................................................................................ 3-3
3.2.1 Main features..........................................................................................................................3-3
3.3 Description........................................................................................................................... 3-4
3.3.1 Patient circuit view..................................................................................................................3-4
3.3.2 Electric connexion view..........................................................................................................3-5
3.3.3 Upper view..............................................................................................................................3-6
3.4 Use ...................................................................................................................................... 3-7
3.4.1 CO2 soda lime absorber canister...........................................................................................3-7
3.4.2 Connections to valves group ................................................................................................3-10
4 DESCRIPTION........................................................................................................4-1
4.1 Introduction.......................................................................................................................... 4-2
4.2 Anaesthesia module with electronic flowmeters box........................................................... 4-3
4.2.1 Main features..........................................................................................................................4-3
4.3 Description........................................................................................................................... 4-6
4.3.1 MENU...................................................................................................................................4-10
4.3.2 Electronic flowmeter box alarm.............................................................................................4-15
4.3.3 Various notes........................................................................................................................4-16
5 LUNG VENTILATOR MODULE .............................................................................5-1
5.1 Ventilator switching on......................................................................................................... 5-2
5.1.1 “ SELF TEST” not passed.......................................................................................................5-5
5.1.2 STAND-BY displaying at the end of “ SELF TEST “ phase ....................................................5-6
5.2 Ventilator switching off......................................................................................................... 5-7
5.3 Monitoring areas and parameters configuration.................................................................. 5-8
5.4 Operative mode area ( A )................................................................................................... 5-9
5.5 Alarms area ( B ) ............................................................................................................... 5-10
5.6 User’s controls area ( C )................................................................................................... 5-11
5.6.1 Operator controls description................................................................................................5-11
5.7 General information area ( D )........................................................................................... 5-13
5.8 Graphics setting area ( E )................................................................................................. 5-14
5.9 Main Menu area ( F )......................................................................................................... 5-16
5.9.1 Main Menu – SETUP............................................................................................................5-17
5.9.2 Main Menu – ALARMS.........................................................................................................5-22
5.9.3 Main Menu – TRENDS.........................................................................................................5-23
5.9.4 Main Menu – EVENTS .........................................................................................................5-26
5.9.5 Main Menu – PATIENT DATA..............................................................................................5-28
5.9.6 Main Menu – PATIENT DATA ERASE.................................................................................5-30
5.9.7 Main Menu – DEFAULT PARAMETERS..............................................................................5-31
5.10 Ventilation parameters setting area ( G ) .......................................................................... 5-32
5.10.1 Operative modes and relevant ventilation parameters .........................................................5-34
5.11 Graphics displaying area ( H - E ) ..................................................................................... 5-38

MORPHEUS XIII
5.12 Ventilation parameters monitoring area ............................................................................ 5-42
5.12.1 Respiratory parameters monitoring ......................................................................................5-42
5.12.2 GAS parameters monitoring.................................................................................................5-43
5.12.3 ADDITIONAL parameters monitoring...................................................................................5-44
5.13 Calibration programs (service area).................................................................................. 5-47
5.13.1 Introduction...........................................................................................................................5-48
5.13.2 Encoder knob .......................................................................................................................5-48
5.13.3 Disable/ Enable turbine (not available).................................................................................5-49
5.13.4 Turbine characterization (not available)................................................................................5-49
5.13.5 Expired flow sensor calibration.............................................................................................5-50
5.13.6 VTEc OFF.............................................................................................................................5-50
5.13.7 High Altitude.........................................................................................................................5-51
5.13.8 Exit .......................................................................................................................................5-51
5.13.9 CALIBRATION PROGRAMS activation................................................................................5-52
6 PREPARATION TO USE........................................................................................6-1
6.1 General warnings ................................................................................................................ 6-2
6.2 Before the use ..................................................................................................................... 6-4
6.2.1 Assembling of O2 cell.............................................................................................................6-4
6.2.2 Assembling of absorber canister ............................................................................................6-4
6.2.3 Battery charger.......................................................................................................................6-6
6.3 Preparation to use ............................................................................................................... 6-8
6.3.1 Medical gas connection..........................................................................................................6-8
6.3.2 Connection of medical gas supply from cylinders...................................................................6-9
6.3.3 Medical gas connection checks............................................................................................6-10
6.3.4 Connection of anaesthetic gases scavenging system..........................................................6-11
6.3.5 Patient circuit connections....................................................................................................6-12
6.3.6 Fresh gases exit – TO and FRO patient circuit.....................................................................6-13
6.3.7 Connection of circuit for manual ventilation..........................................................................6-14
6.3.8 Use of antibacterial filter.......................................................................................................6-15
6.3.9 Gas analyzer connection......................................................................................................6-15
6.3.10 Mains power supply..............................................................................................................6-16
6.3.11 Protection fuses....................................................................................................................6-19
6.3.12 Connection to other equipments...........................................................................................6-20
6.3.13 Table of predisposition sequence for use.............................................................................6-21
6.4 Preliminary tests – Introduction......................................................................................... 6-22
6.4.1 Verification activity - “ SELF TEST “ .....................................................................................6-24
6.4.2 “ SELF TEST “ not overcome ...............................................................................................6-28
6.4.3 “ SELF TEST “ Verifications..................................................................................................6-29
6.4.4 STAND-BY displaying ..........................................................................................................6-30
6.5 Preliminary tests – Operating phase ................................................................................. 6-31
6.5.1 Preliminary checks - TEST ON DEMAND ............................................................................6-32
6.5.2 Preliminary checks – Flowmeter box....................................................................................6-42
6.5.3 Preliminary tests - Lung ventilator ........................................................................................6-47
6.5.4 Preliminary tests - Alarms.....................................................................................................6-49
6.6 Conclusions....................................................................................................................... 6-51
6.7 Preliminary tests sequence table ...................................................................................... 6-52

XIV User’s Manual, version DU3300105
7 VENTILATOR USE .................................................................................................7-1
7.1 Introduction.......................................................................................................................... 7-2
7.2 Flowmeter box..................................................................................................................... 7-3
7.2.1 Dosing and administration of fresh gas ..................................................................................7-3
7.2.2 Administration of fresh gas in the "TO and FRO" system.......................................................7-5
7.2.3 Electronic flowmeter box pre-setting.......................................................................................7-6
7.3 Lung ventilator..................................................................................................................... 7-8
7.3.1 Operative mode selection.......................................................................................................7-8
7.3.2 Respiratory physiological parameters [hereinafter PRF] setting.............................................7-9
7.3.3 DEFAULT PRF parameters setting ......................................................................................7-10
7.3.4 PATIENT DATA setting ........................................................................................................7-12
7.3.5 PATIENT DATA erase..........................................................................................................7-12
7.4 Operative Modes ............................................................................................................... 7-13
7.4.1 STAND-BY ...........................................................................................................................7-14
7.4.2 APCV....................................................................................................................................7-15
7.4.3 APCV-TV..............................................................................................................................7-17
7.4.4 PSV ......................................................................................................................................7-19
7.4.5 VC/VAC................................................................................................................................7-21
7.4.6 VC/VAC BABY......................................................................................................................7-23
7.4.7 SIMV (+PS / SPONT)...........................................................................................................7-25
7.4.8 MAN .....................................................................................................................................7-27
7.4.9 APNOEA BACK-UP..............................................................................................................7-30
7.5 Respiratory physiological parameters [ PRF ]................................................................... 7-31
7.5.1 Description of PRF parameters ............................................................................................7-31
7.6 Monitoring.......................................................................................................................... 7-36
7.6.1 Parameters monitoring.........................................................................................................7-36
7.6.2 Monitoring of curves and loops.............................................................................................7-38
7.6.3 TRENDS...............................................................................................................................7-38
7.6.4 EVENTS...............................................................................................................................7-39
7.7 MAIN MENU...................................................................................................................... 7-40
7.7.1 MENU (first level) .................................................................................................................7-40
7.7.2 MENU (second level)............................................................................................................7-41
8 ALARMS.................................................................................................................8-1
8.1 Definitions............................................................................................................................ 8-2
8.2 General................................................................................................................................ 8-3
8.2.1 Logic on alarm management..................................................................................................8-3
8.3 Displaying and used symbols.............................................................................................. 8-5
8.3.1 Alarm area..............................................................................................................................8-6
8.3.2 ALARMS parameter, MAIN MENU.........................................................................................8-8
8.3.3 General information area......................................................................................................8-10
8.3.4 Acoustic alarm silencing.......................................................................................................8-10
8.4 List of alarms and priorities................................................................................................ 8-11
8.4.1 Lung Ventilator – configurable alarms..................................................................................8-11
8.4.2 system alarms ......................................................................................................................8-12
8.4.3 Gas Sensor (if provided) – configurable alarms....................................................................8-13
8.4.4 Gas Sensor (if provided) – system alarms............................................................................8-14

MORPHEUS XV
8.5 Alarms adjustment............................................................................................................. 8-15
8.5.1 How setting the ALAMRS-MENU entries..............................................................................8-15
8.5.2 How to adjust the alarm volume ...........................................................................................8-16
8.6 Regulations and default values table ................................................................................ 8-18
8.6.1 Lung Ventilator .....................................................................................................................8-18
8.6.2 Gas Sensor (if provided).......................................................................................................8-19
8.7 Description......................................................................................................................... 8-22
8.7.1 Alarms with limits that can be set by the operator ................................................................8-22
8.7.2 System alarms and those that cannot be set by the operator...............................................8-24
8.7.3 Gas Sensor (if provided).......................................................................................................8-27
9 TROUBLESHOOTING............................................................................................9-1
9.1 List of malfunctioning........................................................................................................... 9-1
10 MAINTENANCE....................................................................................................10-1
10.1 Cleaning, disinfection and sterilisation .............................................................................. 10-3
10.2 General instructions........................................................................................................... 10-4
10.2.1 Cleaning ...............................................................................................................................10-4
10.2.2 Disinfection and sterilisation.................................................................................................10-4
10.2.3 Disinfection by immersion (chemical)...................................................................................10-5
10.2.4 Cleaning, disinfection and sterilisation table.........................................................................10-6
10.3 Periodic maintenance......................................................................................................................10-9
10.3.1 Maintenance operations.......................................................................................................10-9
10.3.2 Cleaning, disinfection and sterilization before use with another patient..............................10-11
10.4 Repairs and spare parts ................................................................................................................10-12
10.4.1 Annual kit for MORPHEUS anaesthesia workstation..........................................................10-12
10.5 Storage............................................................................................................................ 10-12
10.6 Repackaging and shipment............................................................................................. 10-12
10.7 Disposal.........................................................................................................................................10-13
11 APPENDIX............................................................................................................11-1
11.1 Technical sheet ................................................................................................................. 11-2
11.1.1 MORPHEUS E - cod. OM3.SE.............................................................................................11-2
11.1.2 Table for Identification of medical gas hose colours.............................................................11-9
11.2 Glossary........................................................................................................................... 11-10
11.3 Electromagnetic compatibility tables ............................................................................... 11-15
11.3.1 Annex A: Table 1................................................................................................................11-15
11.3.2 ANNEX B: Table 2..............................................................................................................11-16
11.3.3 ANNEX C: Table 3..............................................................................................................11-17
11.3.4 ANNEX E: Table 5..............................................................................................................11-17
11.4 Preliminary tests.............................................................................................................. 11-19

XVI User’s Manual, version DU3300105
This page has been left blank intentionally to make front / back copying easier.

MORPHEUS 1-1
1 PRESENTATION
SIARE ENGINEERING INTERNATIONAL GROUP s.r.l. is glad to introduce this new
product, result of 40 years of experience and investment in technological innovation
that we are implementing in recent years.
Siare has focused heavily on innovation of materials, ergonomics and ease of use.
All routine operations have been simplified and the operational procedures are
“foolproof”, in this way there is no margin for the user to make incorrect or inadequate
manoeuvres.
Even the maintenance procedures have been simplified and the parts subject to wear
or deterioration have substantially decreased.
Siare invested much on this project because we firmly believe that it will be a winning
product.
The new anaesthesia unit is considerably different from all previously manufactured
versions: in fact, it can be configurable in different models to respond to the numerous
market demands and requirements.
It starts from a basic anaesthesia unit to arrive at a device that incorporates all
advanced modalities required in modern gaseous anaesthesia, to meet all the
expectations of final users.
1.1 Foreseen use
The MORPHEUS anaesthesia unit is an equipment of new generation, projected for
use in anaesthesia department.
Varying the breathing parameters, adjustable by lung ventilator user’s interface, the
MORPHEUS anaesthesia unit can be used on adults, children, neonatal patients.
The MORPHEUS anaesthesia unit is suitable for the administration of: Oxygen - Air -
Nitrous Oxide - Halothane - Enfluorane - Isoflurane - Sevoflurane - Desflurane
mixtures.
The fraction of inspired oxygen can vary from 21% to 99%.

1-2 User’s Manual, version DU3300105
1.2 Versions
MORPHEUS anaesthesia unit is equipped with a
unique trolley configurable in three different
models to respond to the numerous market
demands and requirements.
The trolley is composed of the following parts:
•mechanic structure of the trolley
•work-shelf and chest
•valves group
•part of electric power supply
The three different models differ for:
•typology of lung ventilator:
•ventilator with basic DISPLAY
•ventilator with advanced 12” TFT display
Flowmeter box typology:
•flowmeter box at 3 GAS 3 ROTAMETERS
•flowmeter box at 3 GAS 5 ROTAMETERS
•ELECTRONIC flowmeter box
1.3 Main technical characteristics
1.3.1 Trolley
The mechanic structure of the trolley is made of light aluminium alloy columns with a
steel base. The pedestal base is made of a shockproof ABS and polyester coated.
This ensures an excellent impact resistance thanks to its flexibility and excellent
abrasion resistance.
Its dimensions and weight are very reduced and allow its installation also in small
rooms or small working areas or combined with pendant lifting systems. The chest is
made of mono-bloc drawers mould in PUR, which are very capacious, rugged and
easy to clean. The drawers are mounted on highly smoothing telescopic guides
allowing a full extension of the drawers.

MORPHEUS 1-3
The work-shelf is mono-bloc, made of a unit PUR mould. The shelf also includes the
housing for the valves group and the manual ventilation controls. The shelf is very
wide and the large handle below the perimeter allows to hold and easily move the
unit.
On the left side a steel rod at full height is provided for fixing the patient monitor and
other accessories like for example the supporting arm for patient circuit, infusion
pumps, etc.
The lateral uprights are provided with a vertical guide for fixing the accessories or
lateral devices, e.g. the pendant lifting system, lateral lectern or PC support for
medical record writing.
On the back side are the medical gas intakes, which are positioned in a rational and
easily visible way. The intake for main supply system and pressure reducer-cylinder
are also provided, with a non return valve and automatic change. In case of main gas
failure just open the cylinder and the gas will be immediately available for use without
additional manoeuvres.
1.3.2 Valves group
Completely renewed and performing, it presents the following definite advantages.
•Extraction from above and perfect integration with the work shelf.
•Automatic connections with double seal gaskets against accidental leaks.
•Mono-bloc, fully sterilizable in autoclave.
•Calibration of flow and O2sensors can be performed in automatic mode by the
operator.
•The access to flow sensor and O2sensor is simple and immediate.
•The CO2absorber canister is located in the upper side and it easy to disconnect
by apposite unlock lever. With canister inserted the system makes automatic
configuration in rebreathing modality; with taken off canister, the system makes
automatic configuration in non rebreathing modality (real open circuit). It is
possible to put and take off the canister during intervention. The canister is
available in version for reuse and disposable version including soda lime
granules.
•Low periodic and extraordinary maintenance operations; easy training of
technical personnel thanks to the extreme rationality of the system and to the
drastic reduction of accidental leaks.

1-4 User’s Manual, version DU3300105
1.3.3 Flowmeter box
The FLOWMETER BOX, is available 3 versions, 2 mechanical (at three or five
flowmeters) and 1 electronic version which foresees the Xenon option.
The mechanical versions are characterized by the complete absence of
mechanical controls; replaced by a small control panel located on the left side of the
work-shelf to manage all manual ventilation operations. This solution allows the
presence of audible and visual alarms of strong impact and without duration limits.
The electronic version is equipped with a wide 5,7” TFT colour monitor which
allows an optimal view and wealth of information. The operator acts with knobs, so
that to facilitate the use also to staff experienced with the traditional mechanical
models. Wide possibility of parameters displaying among: gas supply pressure,
delivered gas flow, delivered O2concentration, fresh gas total flow, consumption
data.
Furthermore the following features are foreseen: a mechanical flowmeter for total flow
control, also backlit; a push-button for selecting the gas to be combined to the oxygen
(N2O, AIR or XENON, this last one is optional).
1.3.4 Lung ventilator - basic DISPLAY
This type of lung ventilator is used on the LT version.
1.3.5 Lung ventilator - ventilator with advanced 12” TFT display
The lung ventilator, through the user’s interface, allows to set and display a wide
range of respiratory parameters useful for the operator for use of MORPHEUS
anaesthesia unit. The lung ventilator version with advanced 12” TFT display foresees
the following standard operative modes: APCV, APCV-TV, PSV, APNEA BACK-UP,
VC-VAC, VC-VAC BABY, SIMV+PS (volumetric) - SPONT, MANUAL.
This lung ventilator type is characterized by a very simple and powerful user’s
interface, with large graphic display of respiratory parameters, the possibility to
choose the curves to be shown simultaneously and an easy interaction in the
selection of menus.
The valves group is automatically configured for the selected modality without manual
procedures, avoiding errors or inadequate manoeuvres. The lung ventilator version
with advanced 12” TFT display foresees the possibility to ad just the end expiration
positive pressure (PEEP), the trigger sensitivity and it is equipped with FiO2
monitoring with automatic calibration and leak test.
This manual suits for next models
3
Table of contents
Other Siare Medical Equipment manuals
Siare
Siare Falco 202 User manual
Siare
Siare Siaretron 4000 User manual

Siare
Siare Falco 101 User manual

Siare
Siare Falco 202 Evo User manual

Siare
Siare AM5000 User manual

Siare
Siare SIARETRON 1000 User manual

Siare
Siare Aria 104 User manual
Siare
Siare Morpheus ND User manual

Siare
Siare Morpheus Series User manual
Popular Medical Equipment manuals by other brands
Cardinal Health
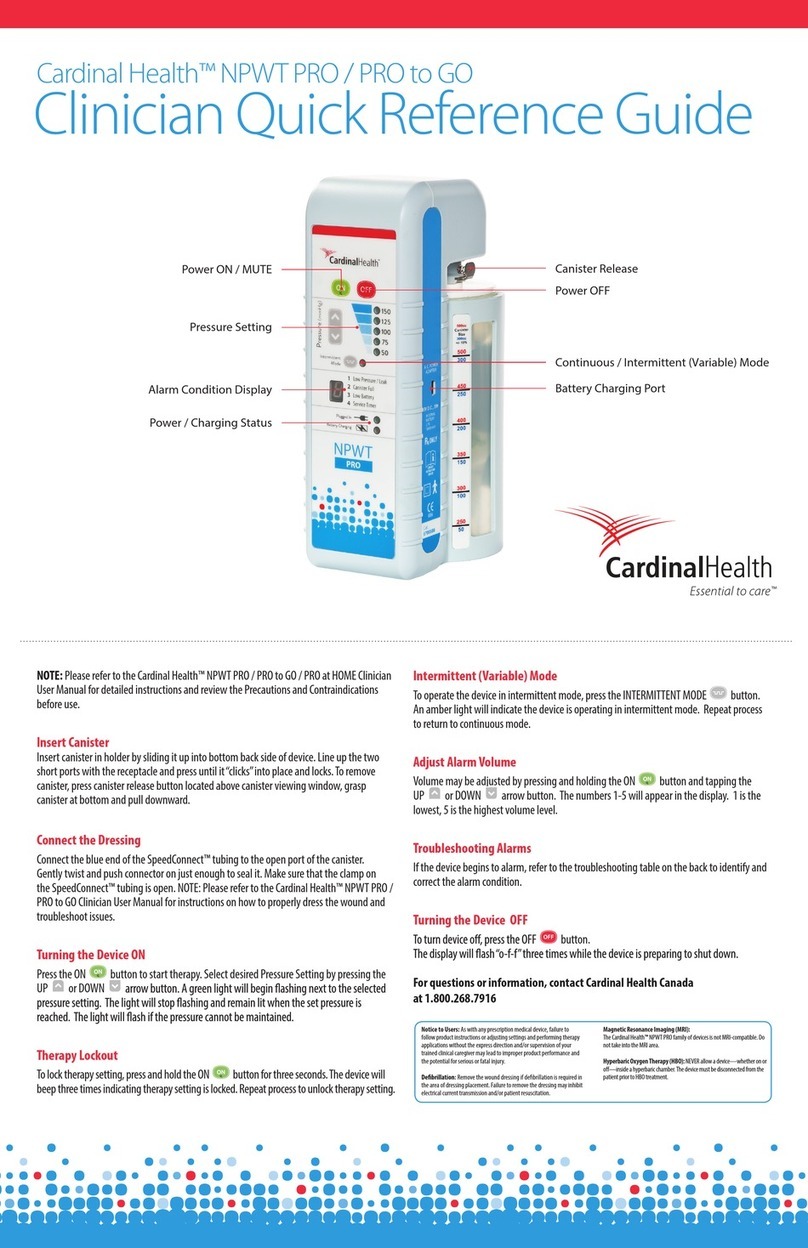
Cardinal Health NPWT PRO Clinician Quick Reference Guide
BTL
BTL BTL-5000 Series Assembly instructions

ResMed
ResMed ELITE S8 Clinician manual

ENHANCED VISION
ENHANCED VISION Smart Reader HD quick start guide

Serene Evolution
Serene Evolution BRRC114 owner's manual

Darco
Darco Body Armor Walker Instructions for use