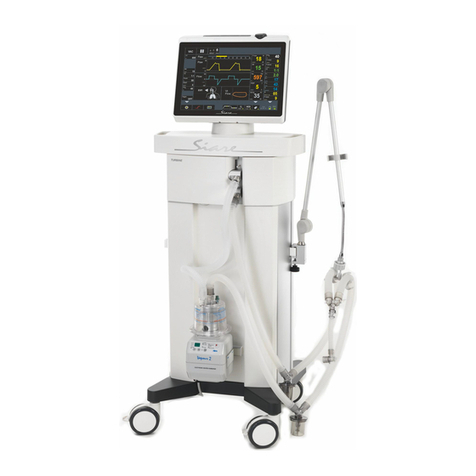
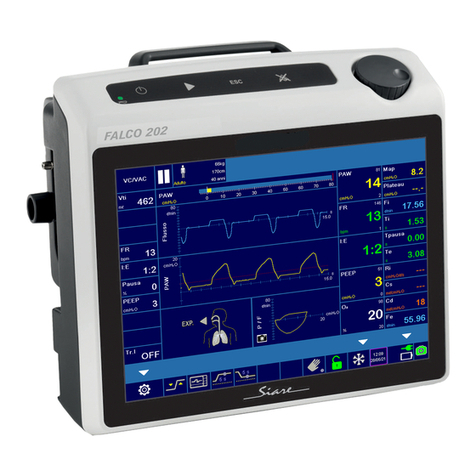
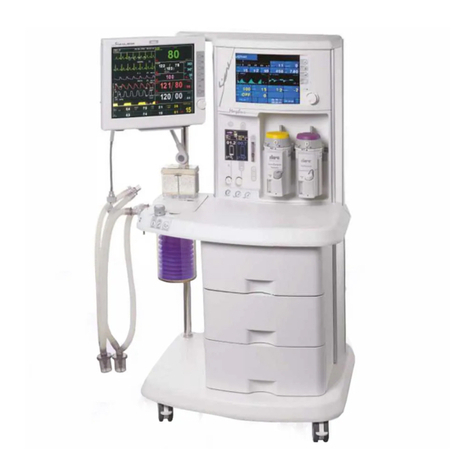
Morpheus_ND V
Warnings, cautions and notes
You are advised to carefully read the information given a
longside the three symbols
shown on the previous page, since it contains considerations on the
requirements for the use of anaesthesia unit and the relative safety regulations.
•In order to understand how the anaesthesia unit works and h
ow to use it correctly
to ensure patient and user safety, the recommendations and instructions contained
in this manual must be read with care and understood.
•In order to grant maximum reliability and to ensure the patient and User
the anaesthesi
a unit was designed and manufactured following warranty standards
of quality of the product and its components. Any part of circuit must therefore only
be replaced with original spare parts supplied or checked by SIARE.
•The anaesthesia unit must only be us
ed for the purposes specified herein and the
safety of the anaesthesia unit is therefore only guaranteed if it is used in
accordance with the instructions given in this manual.
•
The anaesthesia unit must only be used by qualified personnel and only in
equip
ped and dedicated rooms, according to the regulations in force in the country
where the anaesthesia unit is installed. Furthermore, during all the operation of
anaesthesia unit, it is required the presence of qualified personnel.
•Regarding the general safe
ty and to ensure correct technical assistance and avoid
possible physical damage to the patient, the maintenance schedule foreseen in this
manual must be respected; qualified personnel must only carry out maintenance of
the anaesthesia unit or authorised m
odifications to the anaesthesia unit. The user
of this product is solely responsible for any operating defect caused by improper
use or interventions carried out by third parties other than specialised SIARE
personnel.
•The maintenance and the replacement o
f any part have to be performed by
authorized service personnel and only original SIARE spare parts or components
checked by SIARE should be used.
•Regarding the general safety of the electro-
medical equipment, it is important to
follow all rules about the interaction between the machine and the patient, the User
and the nearby environment.
•
For any repairs to anaesthesia unit (due to malfunctioning, defects or failures), the
user must contact SIARE or the authorised local Technical Service Centre; it is
advi
sable to specify the data on the identification label (model, serial number, ……)
when requesting intervention.
•
SIARE recommends establishing a maintenance and service contract with SIARE
or the local authorised service dealer in order to guarantee the sche
maintenance required to operate the anaesthesia unit in a safe and correct manner.
•
To prevent the risk of fire, keep the anaesthesia unit and/or the oxygen tubes away
from matches, lit cigarettes and inflammable material, such as anaesthetic gases
and/or sources of heat.