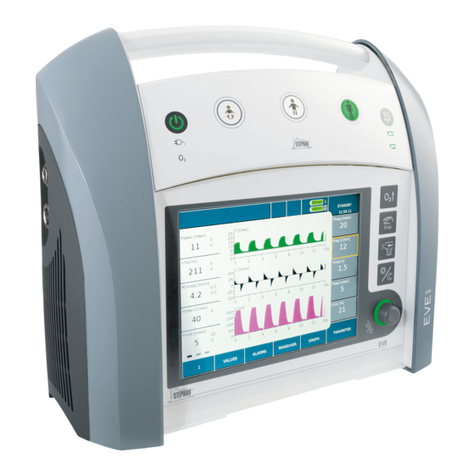
Stephan EVETR User manual

EVETR
Transport Ventilator
Instructions for use

Preface
2
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
This manual aims to provide clear answers to your questions about the
operation and care of the EVETR. It does not contain any information
about repairs, installation or hazards that are clearly observable by the
user or caused by the non-intended use of or non-authorized
modifications to the device.
If any malfunctions occur while operating the device, please contact the
authorized FRITZ STEPHAN GMBH customer service team or the
authorized specialist dealer who supplied the device and familiarized you
with its function and operation.
The manufacturer only guarantees the safety and reliability of the EVETR
when it is operated according to the manual.
NOTE
For information about the maintenance and care of the EVETR, please see the service
manual. This also contains detailed information about the device design and function
as well as its individual components.
NOTE
Fritz Stephan GmbH offers training on the safe and efficient use of the
EVE ventilator. Training material can also be requested. Please contact
info@stephan-gmbh.com for more information.
Fritz Stephan GmbH Equipment is subject to technical modification.
- Medizintechnik -
Kirchstraße 19 Valid as of: May 2018
Version: V2.7
D-56412 Gackenbach From software version: V2.2
Art. no.: 107090011

®
Table of contents
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
3
Table of contents
Table of contents........................................................................................3
1General information...........................................................................9
1.1 Product combination..............................................................10
1.2 Optional components.............................................................12
1.2.1 Software components ................................................12
1.2.2 Hardware components...............................................12
1.3 Device name and manufacturer .............................................12
1.4 Intended use...........................................................................13
1.5 Contraindications...................................................................14
1.6 Disposal .................................................................................14
1.7 Introduction ...........................................................................15
1.8 Abbreviations, definitions, and pictograms ...........................16
1.9 Specifications.........................................................................22
2Safety instructions............................................................................33
2.1 Danger ...................................................................................34
2.2 Warning .................................................................................35
3Design and functional description....................................................41
3.1 Front view..............................................................................41
3.1.1 Alarm indicator .........................................................42
3.1.2 Control panel .............................................................43
3.1.3 Function buttons........................................................46
3.2 Touchscreen monitor .............................................................49
3.2.1 General information about touchscreen navigation...50
Selecting a function field .................................503.2.1.1
Setting options and parameters ........................513.2.1.2
Functions in the System Settings menu ...........523.2.1.3
3.2.2 Measured value display.............................................54
3.2.3 Function fields...........................................................57
Measured value indicator switch......................573.2.3.1
Values ..............................................................573.2.3.2
Alarms..............................................................593.2.3.3
Maneuver .........................................................663.2.3.4
Graph................................................................683.2.3.5

Table of contents
4
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
Parameter .........................................................683.2.3.6
3.2.4 Parameter display ......................................................69
3.2.5 Ventilation mode display ..........................................70
3.2.6 Energy supply indicator ............................................71
3.2.8 Status, alarm and info display ...................................73
3.2.9 Graphic display .........................................................74
Configuring the measurement curves...............753.2.9.1
Configuration of loops and trends....................763.2.9.2
3.3 Left side view ........................................................................79
3.4 Right side view ......................................................................80
3.6 Rear view...............................................................................82
3.7 Brackets .................................................................................83
3.7.1 Ambulance bracket....................................................83
3.7.2 Helicopter bracket .....................................................84
3.7.3 Carry system..............................................................85
Built-in pressure regulator ...............................853.7.3.1
4System Settings................................................................................87
4.1 System ...................................................................................88
4.1.1 Info ............................................................................89
4.1.2 Display ......................................................................89
4.1.3 Time and Date ...........................................................89
4.1.4 Function.....................................................................90
4.2 Sensors...................................................................................91
4.2.1 Pulse oximetry...........................................................91
Settings.............................................................924.2.1.1
Parameter .........................................................934.2.1.2
SpO2.................................................................944.2.1.3
SpHb ................................................................954.2.1.4
4.2.2 Capnometry ...............................................................96
4.2.3 Flow...........................................................................97
4.2.4 Ventilation tube system.............................................98
4.3 Display...................................................................................99
4.3.1 Measured values........................................................99
4.4 Setup ....................................................................................100
4.4.1 Oxygen configuration..............................................101
4.4.2 I:E/Tinsp..................................................................102

®
Table of contents
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
5
4.4.3 Language .................................................................103
4.4.4 Touchscreen calibration ..........................................103
4.4.5 Factory Settings.......................................................103
4.4.6 Service Software .....................................................103
4.4.7 Deep Sleep mode.....................................................104
4.4.8 Sound.......................................................................104
4.4.9 Logbook ..................................................................105
4.4.10 License ....................................................................105
5Preparation for use .........................................................................107
5.1 Connecting the oxygen supply.............................................107
5.1.1 Connecting the oxygen cylinder..............................108
5.1.2 Sample calculation: O2oxygen consumption..........110
5.1.3 Changing the oxygen cylinder.................................111
5.1.4 Connection to the central gas supply.......................112
5.1.5 Connecting to an oxygen concentrator....................112
5.2 Energy supply connection....................................................113
5.2.1 Mains power supply ................................................113
5.2.2 12/24 V mains power supply...................................113
5.2.3 Internal energy supply.............................................114
5.2.4 Charging the external battery ..................................115
5.3 Connecting the patient tube system .....................................115
5.3.1 EVE adults emergency single-use tube system.......116
5.3.2 EVE pediatric single-use tube system.....................117
5.3.3 Configuration for use of the EasyFlow nCPAP system118
5.4 Installing the expiration valve .............................................118
5.4.1 Connecting the distal expiration valve ....................119
5.4.2 Connecting the proximal expiration valve ..............119
5.5 Installing the flow sensor.....................................................119
5.6 Installing the patient filter....................................................120
5.7 Installing the SpO2sensor....................................................121
5.8 Aerosol nebulization............................................................121
6Operation .......................................................................................125
6.1 Test before every start-up ....................................................125
6.1.1 Testing requirements ...............................................125
6.1.2 Test list ....................................................................126
6.2 Switching the device on/off.................................................127

Table of contents
6
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
6.3 Selftest .................................................................................127
6.3.1 Selftest passed .........................................................127
6.3.2 Selftest failed...........................................................128
6.4 Standby mode ......................................................................129
6.5 Using fast tracking keys ......................................................129
6.6 New Patient..........................................................................130
6.7 Ventilation tube system .......................................................131
6.7.1 Default ventilation parameters ................................132
6.8 Mode Settings ......................................................................133
6.8.1 Monitoring...............................................................135
6.8.2 Selecting the ventilation mode ................................136
6.9 Ending ventilation................................................................138
7Ventilation modes ..........................................................................139
7.1 Invasive and non-invasive ventilation modes......................139
7.2 Trigger functionality............................................................142
7.2.1 Flow trigger .............................................................142
7.2.2 Internal flow trigger ................................................142
7.3 Mandatory ventilation..........................................................143
7.3.1 Volume-controlled ventilation ................................144
Volume-controlled continuous mandatory7.3.1.1
ventilation (VC-CMV)...................................144
Volume-controlled synchronized intermittent7.3.1.2
mandatory ventilation (VC-SIMV)................146
7.3.2 Pressure-controlled ventilation modes ....................149
Pressure-controlled continuous mandatory7.3.2.1
ventilation (PC-CMV)....................................149
Non-invasive pressure-controlled mandatory7.3.2.2
ventilation (nPC-CMV)..................................151
Pressure-controlled synchronized intermittent7.3.2.3
mandatory ventilation (PC-SIMV).................152
Non-invasive pressure-controlled synchronized7.3.2.4
intermittent mandatory ventilation (nPC-SIMV)155
Pressure-controlled assist/control ventilation7.3.2.5
(PC-ACV) ......................................................156
Non-invasive pressure-controlled assist/control7.3.2.6
ventilation (nPC-ACV) ..................................157
Pressure-controlled assist/control ventilation +7.3.2.7
(PC-ACV+) ....................................................158

®
Table of contents
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
7
Non-invasive pressure-controlled assist/control7.3.2.8
ventilation plus (nPC-ACV+) ........................158
DUOPAP........................................................1597.3.2.9
Non-invasive DUOPAP (nDUOPAP)............1627.3.2.10
7.3.3 Spontaneous breathing ............................................163
CPAP..............................................................1637.3.3.1
nCPAP............................................................1657.3.3.2
7.3.4 O2therapy................................................................166
7.3.5 High flow O2therapy ..............................................166
7.4 Additional options for ventilation modes ............................167
7.4.1 Pressure-regulated and volume-controlled ventilation
(PRVC)....................................................................167
7.4.2 Pressure support ventilation (PSV) .........................169
7.4.3 Tube compensation .................................................171
8CO2measurement (optional)..........................................................173
8.1 Measurement using the mainstream technique....................174
8.1.1 Intended use.............................................................174
8.1.2 Specifications ..........................................................175
8.1.3 Warnings .................................................................175
8.1.4 Installing the CO2measuring probe ........................178
8.1.5 Running zero calibration .........................................181
8.1.6 Probe status indicator ..............................................182
8.1.7 Cleaning the probe ..................................................182
8.2 Measurement using the sidestream technique .....................182
8.2.1 Intended use.............................................................183
8.2.2 Specifications ..........................................................183
8.2.3 Warnings .................................................................184
8.2.4 Installing the ISA™ CO2analyzer ..........................188
8.2.5 Running zero calibration .........................................190
8.2.6 Status indicator of the analysis adapter ...................190
8.2.7 Cleaning the CO2analyzer ......................................191
9Functional description....................................................................193
10 Troubleshooting .............................................................................195
10.1 List of errors ........................................................................195
10.2 Selftest error ........................................................................208
10.3 Moisture in the PNT B (flow sensor) ..................................209
11 Care and maintenance....................................................................211

Table of contents
8
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
11.1 Disinfection and sterilization...............................................211
11.2 Information about cleaning agents and disinfectants...........212
11.3 Automated cleaning and disinfection ..................................213
11.4 Manual cleaning and disinfection........................................215
11.5 Cleaning and disinfection of device surfaces ......................216
11.6 Sterilization..........................................................................217
11.7 Carrying out the treatment...................................................218
11.7.1 Type B Pneumotachograph (flow sensor) ...............218
Preparing the PNT B ......................................22011.7.1.1
Post-treatment ................................................221
11.7.1.2
11.7.2 Test lung Neo with tube adapter..............................224
11.8 Treatment table ....................................................................226
11.9 Safety checks .......................................................................227
11.10 Maintenance.........................................................................227
11.11 Servicing..............................................................................228
11.11.1Procedure.................................................................230
Replacing the coarse filter .........................23011.11.1.1
Replacing the HEPA filter.........................23111.11.1.2
Replacing the fan filter ..............................23111.11.1.3
Replacing the external battery ...................23311.11.1.4
12 Electromagnetic emissions and immunity .....................................235
12.1 Electromagnetic emissions ..................................................235
12.2 Electromagnetic immunity...................................................236
12.3 Recommended separation distance......................................239
13 Accessory list.................................................................................241
14 Warranty ........................................................................................245
15 List of figures.................................................................................247
16 List of tables...................................................................................251
17 Notes ..............................................................................................253

®
1 General information
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
9
1General information
FRITZ STEPHAN GMBH disclaims any warranty with respect to the
operation of unauthorized device combinations with products not
approved by the manufacturer or without certified compatibility.
ATTENTION
Do not reuse disposable accessories!
The necessary reconditioning may lead to the deterioration of mechanical and
biological product properties, posing a significant risk to the patient. In addition,
reusing such accessories dangerously increases the risk of contamination for the
patient.
ATTENTION
The sole responsibility for selecting a suitable patient monitoring system that provides
reliable data about the correct functioning of the medical device and the condition of
the patient lies with the user of the ventilator. Different techniques can be used to
monitor patient safety, from the electronic monitoring of the correct functioning of the
medical device and the condition of the patient to simple direct observation of the
patient. The sole responsibility for selecting a suitable patient monitoring technique
lies with the user.
ATTENTION
All electrical cables and gas connections connected to the medical device must
comply with applicable standards.
NOTE
The applied parts of the EVE (CO2sensor, SpO2sensor and ventilator breathing
system (VBS)) are protected against defibrillation.
For the SpO2and CO2module, the recovery time is under 5 seconds.

1 General information
10
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
1.1 Product combination
FRITZ STEPHAN GMBH disclaims any warranty with respect to the
operation of unauthorized device combinations with products not
approved by the manufacturer or without certified compatibility.
1. Medication nebulizer
Pneumatic medication nebulizer 22m/22f
Manufacturer: GaleMed
2. Flow sensors
Flow sensor, adults
Manufacturer: Fritz Stephan GmbH
Flow sensor, children
Manufacturer: Fritz Stephan GmbH
Pneumotachograph, preterm infants and newborns, type B
for ventilator EVE
Manufacturer: Fritz Stephan GmbH
Pressure measuring adapter nCPAP
Manufacturer: Fritz Stephan GmbH
3. Disposable patient tube systems
EVE adult emergency
Manufacturer: Fritz Stephan GmbH
EVE paediatrics
Manufacturer: Fritz Stephan GmbH
EVE adult ICU
Manufacturer: Fritz Stephan GmbH
Patient tube system 10 mm, newborn, heated
Manufacturer: WILAmed
Patient tube system 15 mm, child, heated
Manufacturer: WILAmed
Patient tube system 22 mm, adult, heated
Manufacturer: WILAmed
Patient tube system RT 265, child, heated
Manufacturer: Fisher & Paykel
Patient tube system RT 380, adult, heated
Manufacturer: Fisher & Paykel
Patient tube system RT330 Optiflow Junior, heated
Manufacturer: Fisher & Paykel
Ventilation tube RT024
Manufacturer: Fisher & Paykel
Certified product
combinations

®
1 General information
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
11
4. NCPAP patient interfaces
EasyFlow NCPAP system
Manufacturer: Fritz Stephan GmbH
5. Expiration valve
Manufacturer: Fritz Stephan GmbH
CO2 sensor
Manufacturer: Masimo
6. Masimo Rainbow
Manufacturer: Masimo
7. Optiflow Junior Nasal Cannula
Manufacturer: Fisher & Paykel
8. Optiflow + Nasal Cannula
Manufacturer: Fisher & Paykel
NOTE
Further information on the accessories for the ventilator can be found in chapter 13.

1 General information
12
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
1.2 Optional components
1.2.1 Software components
On customer request, EVETR can also be equipped with the following
software components:
License graphic (loops & trends), art. no.: 107061451
License Neo mode, art. no.: 107061460
License Niv / Duopap, art. no.: 107061450
License ACV+/nACV+, art. no.: 107061452
1.2.2 Hardware components
The EVETR can optionally be equipped with a CO2measurement using
the mainstream or sidestream technique (see chapter 8) as well as a pulse
oximeter for measuring the Masimo Rainbow® parameters Pulse, PVI,
PI, SpMet, SpCO, and SpOC (see supplemental pulse oximetry manual).
1.3 Device name and manufacturer
EVETR
Fritz Stephan GmbH
- Medizintechnik -
Kirchstraße 19
D-56412 Gackenbach
+49 (0)6439 9125 –0
+49 (0)6439 9125 –111
info@stephan-gmbh.com
www.stephan-gmbh.com
Device name
Manufacturer

®
1 General information
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
13
1.4 Intended use
The EVETR is used for invasive and non-invasive ventilation in emergency
and transport settings. The EVETR is suitable for long-term ventilation.
The ventilator is available in different feature levels and can be used by
first responders as well as for ground, water, or air transport. The EVETR
is suitable for the ventilation of children and adults weighing up to
200 kg. It is also possible to ventilate preterm infants and newborns.
The EVETR supports the following types of ventilation:
Ventilation mode
Standard
Optional
PC-CMV
X
-
PC-SIMV
X
-
PC-ACV
X
-
PC-ACV+
-
License ACV+/nACV+
required
CPAP
X
-
VC-CMV
X
-
VC-SIMV
X
-
O2therapy
X
-
High flow
O2therapy
-
License Neo mode
required
DUOPAP
-
License Neo mode or
NIV-DUOPAP required
nPCMV
-
License Neo mode
required
nPC-SIMV
-
License Neo mode
required
nPC-ACV
-
License Neo mode
required
nPC-ACV+
-
License ACV+/nACV+
required
nDUOPAP
-
License Neo mode or
NIV-DUOPAP required
nCPAP
-
License Neo mode
required
Tab. 1: Therapeutic scope
Therapeutic scope

1 General information
14
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
1.5 Contraindications
The safety instructions provided in chapter 2 must be observed. No
additional contraindications exist.
It is the sole responsibility of the user to select the most appropriate type
of ventilation based on the patient's medical condition. The continuous
monitoring of the patient's condition must be assured at all times.
Non-invasive ventilation is contraindicated in the following cases:
No spontaneous breathing
Fixed or functional airway obstruction
Gastrointestinal bleeding or ileus
1.6 Disposal
The device packaging largely consists of recyclable or reusable materials.
The cardboard packaging can be reused or disposed of as used paper.
FRITZ STEPHAN GMBH will accept the return of any used devices from
our company free of charge and dispose of these correctly, thus making a
contribution to the environment.
Used batteries and the device itself must not be disposed of as domestic
waste. Proper disposal must be conducted by a certified electrical and
electronic waste recycling company. Disposal via municipal collection
points for waste electrical equipment is not permitted!
WARNING
Risk of explosion!
Do not throw the battery into a fire or open it with force!
NOTE
Before disposing of the device or any of its components, these must be cleaned and
disinfected.
Infectious disposable accessories must be disposed of as specified in the operating
manual!
Disposal of the
packaging
Disposal of the device
and the battery

®
1 General information
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
15
1.7 Introduction
Device setup, operation, and maintenance is only permitted by trained
personnel. All relevant national laws, guidelines, and regulations as well
as the following instructions must be observed:
The device must be operated by trained personnel only. Thorough
knowledge of the operating manual is required.
Only use the device for the intended purpose described in the
operating manual.
Read the operating manual carefully and comply with its
instructions; lasting safety for the patient and user is only ensured
when the device is operated correctly.
The operating manual must be kept readily available at the place
of use.
Inadequate care and incorrect operation can cause downtime and
accidents.
NOTE
The EVETR must be operated from the front side. The operator must have a sufficient
visual angle of the control and display elements.
The manufacturer does not accept any warranty claims resulting from
incorrect operation or inadequate care and maintenance.
The manufacturer only guarantees the safety and reliability of the device
if it is operated in compliance with the operating manual.
Warranty

1 General information
16
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
1.8 Abbreviations, definitions, and pictograms
Abbreviation/
technical
term
Term
Meaning
Battery
Device for storing electrical energy in the
form of chemical energy
Apnea
Respiratory arrest
BTPS
Body Temperature Pressure Saturated
Measuring condition at body temperature,
current ambient pressure and saturated gas
CPAP
Continuous Positive Airway Pressure
Spontaneous breathing with continuous
positive airway pressure.
When breathing under CPAP, the device keeps
the pressure constant on the endotracheal
tube’s connection piece.
Distal
Away from the patient
DUOPAP
Ventilation support at two different pressure
levels
ETT
Endotracheal tube
Exp
Expiration (exhalation)
Time period from the onset of the expiration
flow until the onset of the inspiration flow
Flow limit
Flow limitation
Limitation of the flow for non-invasive
ventilation in NEO mode
HEPA
High Efficiency Particulate Air Filter
High-performance particle filter
HME
Heat and Moisture Exchanger
Heat and moisture exchanger
HP
High priority
An alarm indicating that the user should
intervene without delay. (IEC 60601-1-8)
HW
Notification
IGR
Incremental encoder
Control knob for device operation
Insp
Inspiration (inhalation)
Time period from the onset of the inspiration
flow until the onset of the expiration flow
MP
Medium priority
An alarm indicating that the user should
intervene immediately. (IEC 60601-1-8).

®
1 General information
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
17
Abbreviation/
technical
term
Term
Meaning
nCPAP
Non-invasive continuous positive
airway pressure
Non-invasive spontaneous breathing with
continuous positive airway pressure.
When breathing under CPAP, the device keeps
the pressure constant on the endotracheal
tube’s connection piece.
nPC-CMV
Non-invasive pressure-controlled
mandatory ventilation
Non-invasive pressure-controlled mandatory
ventilation
nPC-SIMV
Non-invasive pressure-controlled
synchronized intermittent mandatory
ventilation
Non-invasive pressure-controlled
synchronized intermittent mandatory
ventilation
nPC-ACV
Non-invasive pressure-controlled –
Assist Control Ventilation
Non-invasive pressure-controlled
assist/control ventilation
nPC-ACV+
Non-invasive pressure-controlled –
Assist Control Ventilation with
expiration trigger
Non-invasive pressure-controlled
assist/control ventilation with expiration
trigger
nDUOPAP
Non-invasive ventilation support at two
different pressure levels
NIST
Non-interchangeable screw thread
Non-interchangeable screw thread
O2
Oxygen level
PC-CMV
Pressure controlled mandatory
ventilation
Pressure controlled mandatory ventilation
PC-SIMV
Pressure controlled synchronized
intermittent mandatory ventilation
Pressure-controlled synchronized intermittent
mandatory ventilation
PC-ACV+
Pressure Controlled –Assist Control
Ventilation with expiration trigger
Pressure-controlled assist/control ventilation
with expiration trigger
PC-ACV
Pressure Controlled –Assist Control
Ventilation
Pressure-controlled assist/control ventilation
PI
Perfusion index
PRVC
Pressure regulated volume control
Pressure-controlled and volume-regulated
ventilation
PSV
Pressure support ventilation
Pressure support ventilation
PVI™
Pleth Variability Index
Plethysmographic variability index
PEEP
Positive End Expiratory Pressure
Positive end expiratory pressure
Pinsp
Inspiratory pressure

1 General information
18
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
Abbreviation/
technical
term
Term
Meaning
PAW
Airway pressure
Pmean
Mean airway pressure
PNT
Pneumotachograph
Flow sensor
Proximal
Close to the patient
Resistive
Creates a pneumatic resistance
SIMV
Synchronized Intermittent Mandatory
Ventilation
Form of ventilation synchronized to the patient
SpCO®
Carboxyhemoglobin measurement
Index for CO level in arterial blood
SpMet™
Methemoglobin measurement
Index for methemoglobin level in arterial
blood
SpHb®
Hemoglobin measurement
Index for hemoglobin level in arterial blood
SpOC®
Oxygen level measurement
Index for oxygen level in arterial blood
Standby
The device is ready for use
STPD
Standard temperature, pressure, dry
Measuring condition at standard temperature
(20 C), standard pressure (1013 mm Hg
absolute), dry
TA
Technical alarm
TC
Tube compensation
Tube compensation
V´
Flow
Volume flow
VC-CMV
Volume-controlled continuous
mandatory ventilation
Volume-controlled continuous mandatory
ventilation
VC-SIMV
Volume-controlled synchronized
intermittent mechanical ventilation
Volume-controlled synchronized intermittent
mechanical ventilation
PU
Packaging unit
VT
Tidal volume
Breathing volume
VTe
Expiratory breathing volume
Expiratory tidal volume
CGS
Central gas supply
V
Volume
Tab. 2: Abbreviations and technical terms

®
1 General information
Fritz Stephan GmbH
GA-070T-0518V2.7-HAO-EN
19
Pictogram
Meaning
Standard ventilation parameter for premature infants and newborns
(see chapter 3.1.2)
Standard ventilation parameter for children (see chapter 3.1.2)
Standard ventilation parameter for adults (see chapter 3.1.2)
O2
O2supply indicator (see chapter 3.1.2)
Mains power indicator (see chapter 3.1.2)
Charge indicator for battery 1 (see chapter 3.1.2)
Charge indicator for battery 2 (see chapter 3.1.2)
Inspiration hold (see chapter 3.1.3)
Pre-oxygenation (see chapter 3.1.3)
Activate aerosol nebulization (see chapter 3.1.3)
Day/Night toggle switch (see chapter 3.1.3)
Lock/unlock touchscreen (see chapter 3.1.3)
Scroll (see chapter 3.2.3)
Return (see chapter 3.2.1.3)
Save settings (see chapter 3.2.1.3)
Ventilation settings for adults pre-selected (see chapter 3.2.5)
Ventilation settings for children pre-selected (see chapter 3.2.5)
Ventilation settings for preterm infants and newborns preselected
(see chapter 3.2.5)
Non-invasive ventilation (see chapter 3.2.5)
Invasive ventilation (see chapter 3.2.5)

1 General information
20
GA-070T-0518V2.7-HAO-EN
Fritz Stephan GmbH
Pictogram
Meaning
PNT (see chapter 3.2.5)
Alarm history contains unacknowledged alarms (see chapter 3.2.8)
Alarm suppression switched on (see chapter 3.2.8)
Pre-oxygenation activated (see chapter 3.2.8)
Aerosol nebulization activated (see chapter 3.2.8)
Touchscreen locked (see chapter 3.2.7)
Mains power (see chapter 3.2.6)
Charge level for battery 1 (see chapter 3.2.6)
Charge level for battery 2 (see chapter 3.2.6)
Contains conventional or rechargeable batteries and must not be disposed of as
domestic waste.
DC
Protective conductor
Ethernet port
Type BF applied part, defibrillator-protected
The instructions in the operating manual must be observed
Read operating manual
Equipotential bonding
Hazard symbol for ESD-sensitive parts
Push lever up to unlock
Manufacturer
Manufacturing date
Serial number
Table of contents
Other Stephan Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Boscarol
Boscarol OB 2012 FA user manual

Clinic6
Clinic6 Freezpen 8g user manual

Handicare
Handicare SystemRoMedic StretcherSling Disposable manual

Medical WAVE
Medical WAVE D-ACTOR 100 Ultra Quick reference guide

MEDICSIGHT
MEDICSIGHT MEDICCO2LON user manual

Posey
Posey Twice-As-Tough 2795 Application Instructions