1 General information
This operating and user manual applies to the PDCare 904 nm Laser by SYMBYX. This laser device is manufactured for SYMBYX
Pty Ltd of Australia by Spectro Analytic Irradia AB of Sweden. (Note: Irradia also manufacture an identical product called the
MIDCARE 904 nm).
Please read this manual before using the product. The reader is advised to keep the manual at hand for future reference when
necessary. MEDICAL ELECTRICAL EQUIPMENT requires special precautions regarding EMC and need to be installed and put into
service according to the EMC information provided in the ACCOMPANYING DOCUMENTS. Portable and mobile RF communication
equipment can affect MEDICAL ELECTRICAL EQUIPMENT.
“WARNING: Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be
used no closer than 30 cm (12 inches) to any part of the PDCare 904 nm Laser by SYMBYX, including cables specified by the
manufacturer. Otherwise, degradation of the performance of this equipment could result.”
The use of accessories, cables, and transducers other than those specified herein as replacement parts for internal components, with
the exemption of transducers and cables sold by the manufacturer of the EQUIPMENT, may result in increased EMISSIONS or
decreased IMMUNITY of the EQUIPMENT. The EQUIPMENT should not be used adjacent to, or stacked on top of, other equipment.
If it is necessary to use the EQUIPMENT under those circumstances, the EQUIPMENT should be continuously observed in order to
verify normal operation in the configuration in which it is used. The RESPONSIBLE ORGANIZATIONS are advised to carry out all
adjustments and cleaning and disinfection PROCEDURES as specified herein. The RESPONSIBLE ORGANIZATIONS are reminded
that the assembly of ME SYSTEMS, as well as modifications during the actual service life, is evaluated as regards to the requirements
of IEC60601-1.
Many countries have regulations, laws, requirements, and standards for personal protective equipment and the installation and use of
lasers, including their clinical use. Contact the appropriate national agency for the correct user requirements.
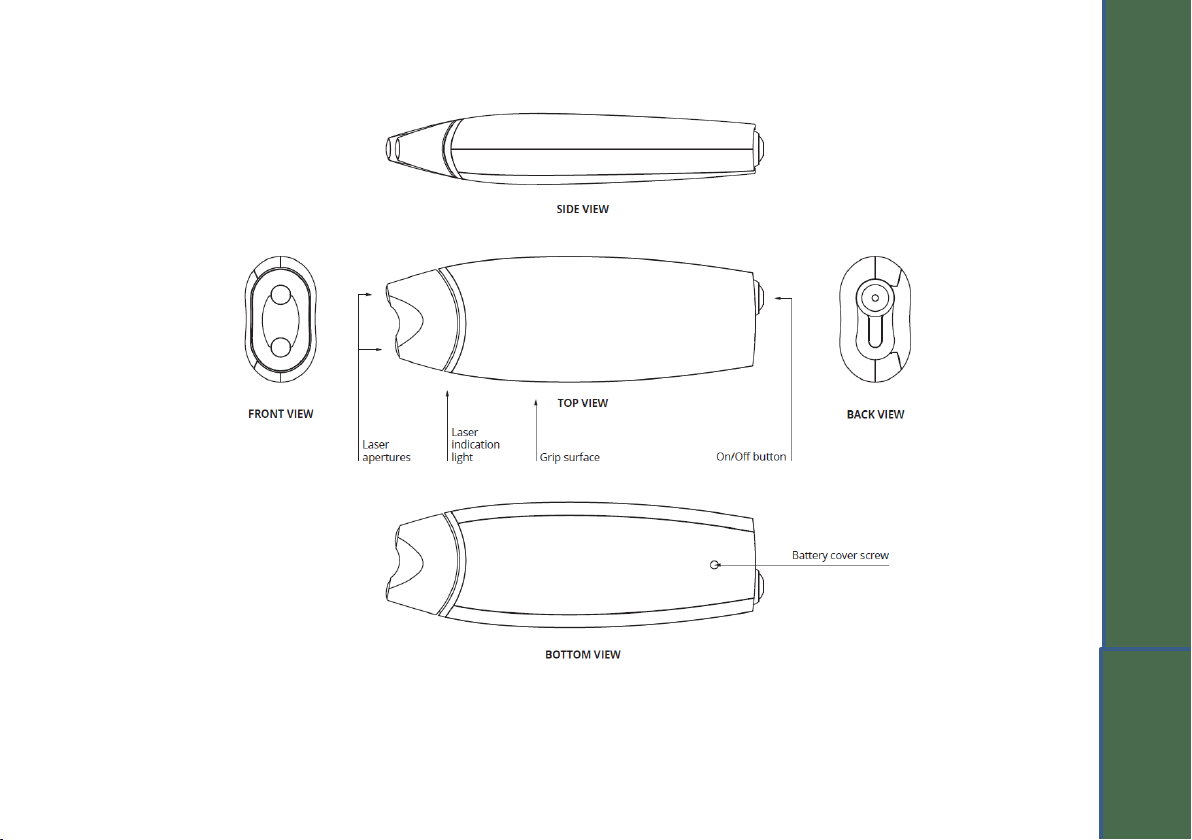
1.1 Product description
This product is a TRANSPORTABLE, HAND-HELD and INTERNALLY POWERED MEDICAL ELECTRICAL EQUIPMENT that is a
NON-INVASIVE CLASS IIa ACTIVE THERAPEUTIC DEVICE, with which the OPERATOR administers TRANSIENT PULSED CLASS
1 INFRARED A (IRA) (model 904) LASER radiation to a PATIENT. The HAND-HELD device is classified as a TYPE BF APPLIED
PART, and IP22. The equipment is classified for CONTINUOUS OPERATION in NORMAL USE.