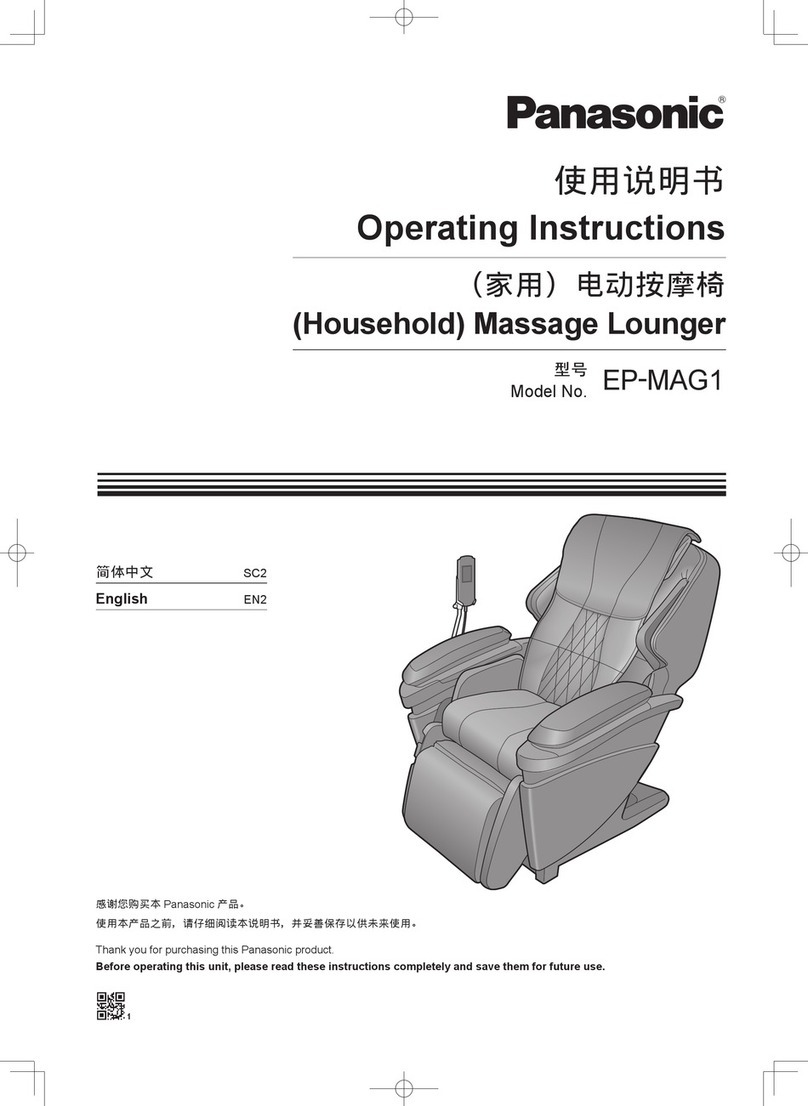
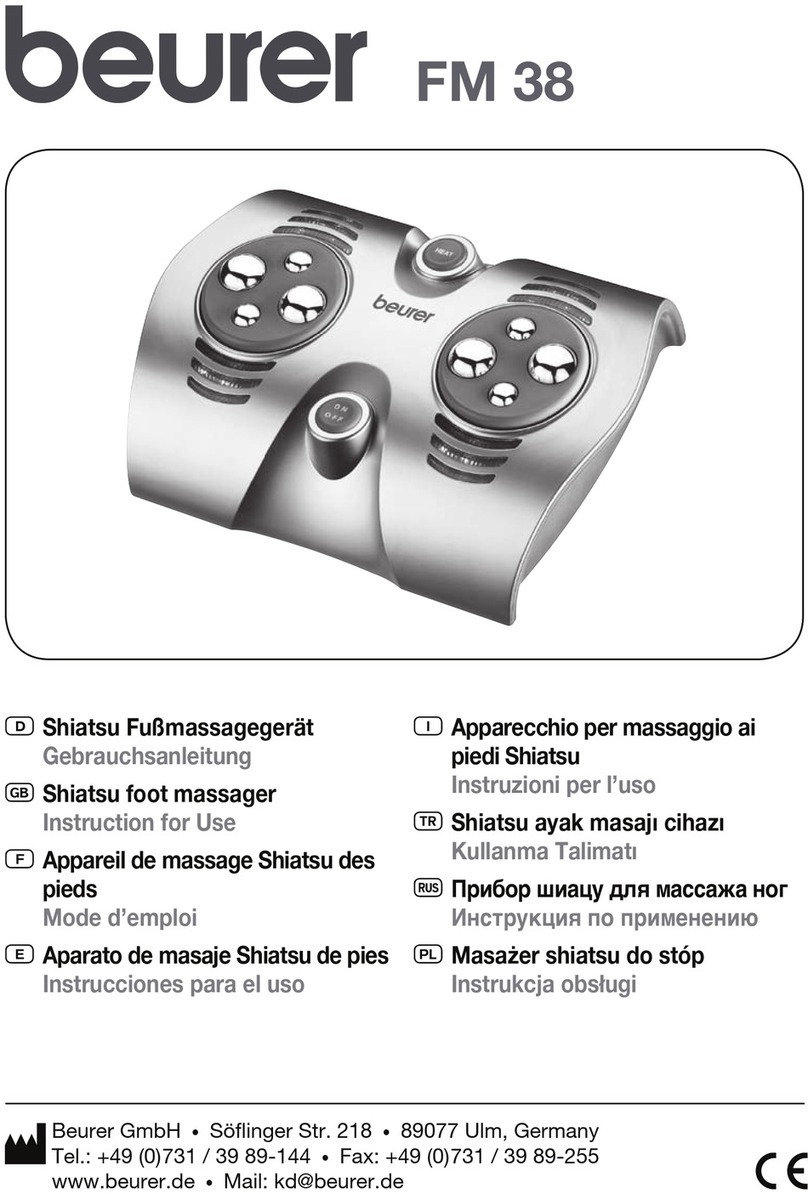
THERAGUN Halo User manual

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
1
Rev History
Modificationdate
Change description
Rev
Modified by
12-4-2021
Update intended use
3
Team
12-4-2021
Updated language and fixed minor
errors
4
Team

User Manual
2
Contents
1 Recommendation of Use 3
1.1Contraindications 4
1.2 Warnings 4
1.3 Precautions 6
1.4 Adverse Reactions 7
1.5 Label & Symbols 7
2 Intended Use & Indications for Use 7
3 Product Description 8
3.1 Detachable component List 10
3.2 Function Introduction 12
3.3 How to Operate the Theragun halo 12
3.3.1 Main device with attachment 13
3.3.3 Keyboard 14
3.3.4 Buttons icon 14
3.3.5 Using Instructions 16
3.3.6 APP instructions(ISO/Android)19
4 Product specification 33
4.1 Switch off the device 34
4.2 Charging 34
5 Maintenance 35
5.1 Error! Bookmark not defined.35
5.2 Trouble Shooting 36
5.3 Disposal of Electrical and Electronic Equipment Waste (E-waste)
6 Service 36
7 Safety, EMC & Biocompatibility 37
8 Manufacturer Information 41
9 LIMITED WARRANTY 41

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
3

User Manual
4
1 Recommendation of Use
1.1 Contraindications
It means that the device is unit for use in certain diseases, conditions, or
specific populations.
1) Never use this device over any damaged tissue or other type of skin condition
can result in delaying the best medical treatment.
2) Do not use if you have had any facial surgery or other surgical procedure –
consult your doctor
3) Do not use if you have a history of epilepsy or seizures
4) Do not use if you are pregnant
5) Never use the device to the patient with Implant Electronic Devices (such as
cardiac pacemaker)
6) The device should not be used in the following areas
- Breast area
- Eye area(circular muscle within the orbital rim)
- Mid-line of neck (bone of neck)
- Groin area
7. The ME EQUIPMENT shall not be serviced or maintained while in use with a
PATIENT.
1.2 Warnings
The patient is an intended operator. The patient can use and maintain the device
and its accessories according to this manual
It’s used to identify a hazard that may lead to death or serious injury.
Do not apply over the eyeballs in the stimulation mode, otherwise will damage the
eyes.
People with a facial trauma are suggested to consult the doctor before using the
Halo device.

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
5
People who have had an aesthetic surgery or had Botox injections should consult
the doctor before using, otherwise will cause physical damage.
Do not use if you have had any facial surgery or other surgical procedure –consult
with your doctor.
Stop using when you have any discomfort.
Use this device only for its intended use as described in this user manual and avoid
causing physical damage.
The device should not be used by children and pregnant women and should be kept
out of the reach of children (0 to 12 years old).
This product is not waterproof, only clean with a moist washcloth wipe the
device .Don't put the device under water.
Do not apply the device near any devices with Electromagnetic Interference (EMI),
such as cell phones, Magnetic Resonance Imaging (MRI), computerized axial
tomography (CT), diathermy, Radio Frequency Identification (RFID), etc. or MR
environment. EMI, RF devices or MR environment may affect the normal function of
the device or would cause user injury.
The devise is for single patient use.
For device with micro current stimulation mode:
If you are being treated by a physician, consult with your physician before using this
device.
Do not apply the electrodes near the thorax; the application of electrodes near the
thorax may increase the risk of cardiac fibrillation.
Do not apply Micro current over your neck because this could cause severe muscle
spasms resulting in closure of your airway, difficulty in breathing, or adverse effects
on heart rhythm or blood pressure.
Do not apply microcurrent across your chest because the introduction of electrical
current into the chest may cause rhythm disturbances to your heart, which could be
lethal.
Do not apply stimulation over painful areas. If you have painful areas, you should
consult with your physician before using this device.
Do not apply stimulation over open wounds or rashes, or over swollen, red, infected,
or inflamed areas or skin eruptions (e.g., phlebitis, thrombosis, varicose veins).
Do not apply stimulation in the presence of electronic monitoring equipment (e.g.,
cardiac monitors, ECG alarms), which may not operate properly when the electrical
stimulation device is in use.
Commented [TP1]: Define “children”
Commented [JS2]: Deleted for adult use only. Need to
discuss

User Manual
6
Simultaneous connection of a user to a high frequency surgical ME equipment may
result in burns at the site of the simulator electrodes and possible damage to the
simulator.
Operation in close proximity (e.g.0. 1 m) to a shortwave or microwave therapy ME
equipment may produce instability in the simulator output.
Do not use the device in the shower.
Do not apply stimulation while sleeping.
Do not use the device on children under 12 years of age.
Do not apply stimulation while driving, operating machinery, or during any activity in
which electrical stimulation can put you at risk of injury.
Consult with your physician before using this device, because the device may cause
lethal rhythm disturbances to the heart in susceptible individuals.
Apply stimulation only to normal, intact, clean, healthy skin.
Keep clean in use, store in a dry place, do not expose to the sun
This appliance contains batteries that are non-replaceable
Avoid the LED light irradiate into your eyes when operate the device with LED ring;
Otherwise it will cause eyes injury.
During the light irradiation treatment, close your eyes to avoid the light beam get into
your eyes.
FCC compliance statement
This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) this device may not cause harmful interference, and (2)
this device must accept any interference received, including interference that may
cause undesired operation.
Changes or modifications not expressly approved by the party responsible for
compliance could void the user’s authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference in a residential
installation. This equipment generates, uses and can radiate radio frequency energy
and, if not installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation.
If this equipment does cause harmful interference to radio or television reception,
which can be determined by turning the equipment off and on, the user is
encouraged to try to correct the interference by one or more of the following
measures:

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
7
-- Reorient or relocate the receiving antenna.
-- Increase the separation between the equipment and receiver.
-- Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
-- Consult the dealer or an experienced radio/TV technician for help.
FCC Radiation Exposure statement
This equipment complies with FCC radiation exposure limits set forth for an
uncontrolled environment.
1.3 Precautions
It’s used to identify a hazard that may result in minor or moderate injury to the user or patient or
damage to the equipment or other property
Stop immediately if the device shows any sign of failure.
(For micro current stimulation treatment mode)
The long-term effects of electrical stimulation are unknown.
Since the effects of stimulation of the brain are unknown, stimulation should not be applied across
your head, and electrodes should not be placed on opposite sides of your head.
The safety of electrical stimulation during pregnancy has not been established.
You may experience skin irritation or hypersensitivity due to the electrical stimulation or electrical
conductive medium (gel).
If you have suspected or diagnosed heart disease, you should follow precautions recommended by
your physician.
If you have suspected or diagnosed epilepsy, you should follow precautions recommended by your
physician.
Use caution if you have a tendency to bleed internally, such as following an injury or fracture.
Consult with your physician prior to using the device after a recent surgical procedure, because
stimulation may disrupt the healing process.
Use caution if stimulation is applied over areas of skin that lack normal sensation.
Keep this device out of the reach of children
Use this device only with the accessories recommended by the manufacturer.
This product is not waterproof, only clean with a moist washcloth wipe the device .Don't put device in
water, otherwise the device will be damaged.

User Manual
8
1.4 Adverse Reactions
1) You may experience headache and other painful sensations during or following the application of
electrical stimulation near your eyes and to your head and face.
2) You may experience skin irritation and burns beneath the stimulation electrodes applied to your
skin.
1.5 Label & Symbols
The following labels and symbols appear on Theragun halo
No.
Symbols
Description
1
CE mark
2
the Restriction of Hazardous Substances
3
“WEEE (Waste Electrical and Electronic Equipment)”.
The waste products should be handled legally.
4
IP22
IP Classification: The first number 2: Protected against
access to hazardous parts with a finger, and the jointed
test finger of 12 mm Ф, 80 mm length, shall have
adequate clearance from hazardous parts, and protected
against solid foreign objects of 12.5 mm Ф and greater.
5
Please read the user manual before use
6
Type BF apply part.
UKCA Mark
2.0 Intended Use & Indications for Use
The device provided with 5 operating modes with its individual intended use as below:

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
9
1) The EMS mode is indicated for facial stimulation;
2) The red light is intended for the treatment of periorbital wrinkles
3) The blue light is intended for the treatment of the mild to moderate inflammatory acne
4) Red+ IR : Indicated to emit energy in the red and IR region of the spectrum for use in
dermatology for the treatment of periorbital wrinkles.
5) Vibration : the mode is intended used for to relax facial muscles
And it is only used for adults.
Target treatment area:
1) Facial stimulation: Both sides of cheek
2) Periorbital wrinkles treatment: Periorbital region
3) Treatment of mild to moderate acne: Both sides of cheek

User Manual
10
3.0 Device Description
The Device provided multiple function modes: Vibration, Micro current stimulation mode, LEDs photo-
therapy mode (Red light, Blue light ,Red +IR). There are Micro current electrodes, Red LEDs, Blue
LEDs,Red +IR LEDs. They can be selected by key button of mode.
Vibration Feature
Select which vibration attachment you would like and attach. Once attached to the Theragun Halo
main device , turn on the vibration feature by short pressing the vibration button once.
To toggle between the 3 speed options (1750, 2100 and 2400 rpm), press the vibration button again.
To stop the vibration, press the vibration button a third times.
For Micro current stimulation mode:
The device has two pairs of electrode contacts for facial stimulation by applying an electrical micro
current to electrodes, which are attached to the user’s facial skin. The output waveform is regulated
Voltage of Pulsed Biphasic and provided with 3 steps of output intensity, which can be adjusted by the
user.
In this operating mode, the device allows the user to adjust setting output intensity from level 1 to
level 3. You can adjust the intensity according to your feeling and response intensity to muscle
contracting.
User can buy the conductive gel individually which are legally sold, to reduce the impedance
between the skin and the stimulating device
For LEDs photo-therapy function:
The device also can provide specific photon spectrum by LED lamps for Red light irradiation mode
and Blue light irradiation mode. There are Red LED lamps and Blue LED lamps assembled in the
treatment head. The red light wavelength is 633±10nm and it is intended for the treatment of
periorbital wrinkles. The blue light wavelength is 415±10nm and is used for the treatment of acne.
The output wavelength of Red+IR:633 ±10nm/830nm±10nm is intended for use in the treatment of
facial wrinkles
In Red light irradiation mode, the device utilizes Light Emitting Diodes to provide red LED light to the
body. The red-light output is a visible light source of high spectral purity. They provide uniform or “hot-

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
11
spot” free illumination. The output is pre-tuned to one wavelength with a narrow spectral bandwidth.
The output wavelength of Red is 633±10 at 73 +/-5mW. During the treatment, the user holds the
device and place treatment head to the periorbital area.
In Blue light irradiation mode, the device utilizes Light Emitting Diodes to provide blue LED light to the
face. The blue light output is a visible light source of high spectral purity. They provide uniform or “hot-
spot” free illumination to face skin. The output is pre-tuned to one wavelength with a narrow spectral
bandwidth. The output wavelength of blue is 415 ±10nm at 50+-5 mW. During the treatment, the user
holds the device and place treatment head to the target treatment area with mild or moderate acne.
In Red+Near-infrared mode is intended for use in the treatment of facial wrinkles,The output
wavelength of Red+IR:633 ±10nm/830nm±10nm at 73+/-5 and 55+/-5. During the treatment,
Indicated to emit energy in the red and IR region of the spectrum for use in dermatology for the
treatment of periorbital wrinkles.
3.1 Detachable component List
Detachable
component
QTY
Materials
Figure
LED Ring
1
Polycarbonate
Micro
current
1
Polycarbonate+
Steel
Commented [TP3]: Conductive gel was mentioned above.
Should be included in the accessory list.
Commented [4R3]: Revised:User can buy the
conductive gel individually which are legally sold, to
reduce the impedance between the skin and the
stimulating device
Commented [JS5R3]: Confirm that we can include the
MC ring but not the MC gel?

User Manual
12
Vibration
massage
head
4
Silicon + EVA
sponge
Silicon
Silicon+Sponge
Pouch
1
ploy urethane
USB cable
1
Polyvinyl chloride
Dummy
stand
1
Polycarbonate
Apply parts are LED Ring, micro current head and vibration massage head.

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
13
3.2 Function Introduction
Vibration Mode:
This mode with 3 vibration levels (1750, 2100 and 2400 rpm) are intended used for to relax facial
muscles
LED photo-therapy Mode:
Red light and blue light functional ,and Red +IR mode:
The red light wavelength is 633±10nm and it is intended for the treatment of periorbital wrinkles.
The blue light wavelength is 415±10nm and is for the treatment of mild to moderate acne.
Red +IR wavelength is 633 ±10nm /830nm±10nm
Choose the red or blue light irradiation or red +IR mode
Micro Current stimulation mode:
There are 3 steps of micro current output intensity, intended for facial stimulation and is indicated for
over-the-counter aesthetic use.
For the facial stimulation model, the output intensity is gradually increased from the minimum output
intensity to obtain a most suitable output intensity.
The user can choose the suitable treatment parameters according to their symptom severity.
3.3 How to Operate the Theragun halo
Using the Theragun halo
Start by washing your face completely and rinse twice to remove all
residue. Allow skin to dry completely before starting treatment.

User Manual
14
3.3.1 Main device with attachment
Figure 3-1. Main Device with attachment
3.3.2 How to use attach
All attachment was magnetic connection to the device and can be removed by pulling, Use alignment indicator to
connect the attachment into the main device
Figure 3-1-1. LED ring attachment
Figure 3-1-2. Micro attachment
Vibration head attachment
Micro current attachment
Mainly device
Red/Blue/Red+IR attachment
LED ring Alignment indicator
Micro current ring Alignment indicator

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
15
Figure 3-1-3. Vibration attachment
3.3.3 Keyboard
Figure 3-2-1. Control Buttons of Main Device
3.3.4 Buttons icon
Power slider button
Vibration button
Control Button for LED / or control button for Micro current
Vibration magnetic connection
ON/Bluetooth/OFF button
Vibration button
OLED screen
LED button/Micro current button
button

User Manual
16
OLED display
Vibration button level 1
Vibration button level 2
Vibration button level 3
MC button Level 1
MC button Level 2
MC button Level 3
LED Red mode
LED Blue mode
LED Red+IR mode
Battery capacity 100%
Battery capacity 75%

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
17
Battery capacity 50%
Battery capacity 25%
Bluetooth
Figure 3-3-1. Buttons icon
3.3.5 Using Instructions
Haptic feedback = vibration motors stops and moves twice in the period of 1sec to let the user know he/she is
supposed to change to a different face region.
Shut-off feature = attachment rings turns off. The device also creates a double haptic feedback to let the user
know the treatment is finished.
1) Vibration mode
1.Connect the vibration head into main device
Figure 3-4-1-1. Vibration attachment

User Manual
18
2. How to use vibration attachment
Figure 3-4-1-2. control panel
Push the Power slider button up, device power ON, Bluetooth broadcasting
Short press the vibration button one time vibration working on Level I , screen display
Short press vibration button one time, vibration working on Level II , screen display
Short press vibration button one time, vibration working on Level III , screen display
Short press vibration button one time ,vibration stop working , screen display back to main screen
Vibration treat times
Freestyle mode-no Haptic Feedback or shut off
1 min per face quadrant
4 min per session.
2) LED mode (Red/Blue/IR+Red mode)
1.Connect the LED ring head into main device
Figure 3-4-2-1. LED ring attachment
Power slider button
Vibration button

Document Name
User manual
Document No.
003-HALO-TG
Model
Theragun halo
Rev
40
19
Figure 3-4-2-2. control panel
Push the Power slider button up, device power ON, Bluetooth broadcasting
Short press LED ring button one time, LED ring working on RED LED mode , screen display , and LED ring light up in red
dimly means LED ring is ready , the LED light up in RED when LED ring touch face skin during working.
Short press LED ring button again,LED ring working on BLUE LED mode , screen display , and LED ring light up in blue
dimly , means LED ring ready , LED light up in blue when LED ring touch face skin during working.
Short press LED ring button again, LED ring working on RED+IR , screen display , and LED ring light up in purple
dimly , means LED ring ready , LED light Red+IR light up red when LED ring touch face skin during working.
Short press LED ring button once again , LED ring stop working , screen display back to main screen
LED Treat time
2 min per face quadrant
8 min per session
2 times per week for 6 weeks
Haptic feedback every 2 min, shut of at 8 min
3) Micro current mode
1.Connect the micro current into main device
Figure 3-4-2-1. micro current attachment
Power slider button
LED button

User Manual
20
Figure 3-4-2-2. control panel
Push the Power slider button up, device power ON, Bluetooth broadcasting
Short press Micro current button one time, device working on Level I , screen display
Short press Micro current button one time , device working on Level II , screen display
Short press Micro current button one time, device working on Level III , screen display
Short press Micro current button one time, device stop working , screen display back to main screen
Automatic Shut Off
the device will automatically shut off after using 8 minutes.
3.3.6 APP instructions(ISO/Android)
The App must be open on your phone in order to establish a connection with the
device Bluetooth® must be turned ON on your smart phone
1Permission setting
After the app is installed, you need to set the permissions manually when you start
the app for the first time. (skip this step if it is not started for the first time).
Micro current button
Power slider button
Commented [I6]: add
Table of contents
Other THERAGUN Massager manuals

THERAGUN
THERAGUN G2PRO User manual

THERAGUN
THERAGUN RecoveryAir User manual

THERAGUN
THERAGUN Elite User manual

THERAGUN
THERAGUN Wave Duo User manual

THERAGUN
THERAGUN Elite User manual

THERAGUN
THERAGUN PRO User manual

THERAGUN
THERAGUN Elite User manual

THERAGUN
THERAGUN mini User manual

THERAGUN
THERAGUN Prime User manual
THERAGUN
THERAGUN PRO PLUS User manual