THERAGUN PRO PLUS User manual

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 1 of 24
User Manual
Name Date
Prepared by: Ice Zheng
Reviewed by: Felix Chen
Approve by: CJ Frederick
Revision History
Modification date Change description Rev Modified by
2023-4-24
Initial release.
1 Team

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Table of Content
1. Recommendation of Use..............................................................................................3
1.1 Contraindications...............................................................................................4
1.2 Warnings............................................................................................................5
1.3 Precautions...........................................................................
1.5 Label & Symbols.....................................................................................................9
2. Intended Use & Indications for Use............................................................................10
3. Device Description ......................................................................................................10
3.1 Smart Features................................................................................................11
3.2 Attachments.....................................................................................................11
3.3 Getting started .................................................................................................12
3.4 Using the Theragun PRO PLUS .................................................................13
3.5 Connecting the attachments............................................................................14
3.6 Charging the Theragun PRO PLUS............................................................14
4. Cleaning Instructions...................................................................................................16
5. Service ........................................................................................................................16
6. Product Specification ..................................................................................................17
7. Safety, EMC ................................................................................................................18
8. Manufacturer Information ............................................................................................24
9. LIMITED WARRANTY.................................................................................................24
............
............
6
Page 2 of 24

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 3 of 24
1. Recommendation of Use
Device name :Theragun PRO PLUS model : Theragun PRO PLUS
Read the full Warnings and Guidance prior to using the Theragun PRO PLUS.
The Theragun THE DEVICE is equipped with 5 buttons and a color LCD screen,
which acts as the main user interface between user and device. These are
located on the top of the device’s head in front of the top handle. This allows the
user to view the screen while controlling the device with a single hand in most
scenarios.
The second point of interaction is the attachments. Users can change the
therapy the device provides by replacing these accordingly (Percussive, Cold,
Heat and Vibration at the moment of Launch). The attachments need to be
physically changed by pulling from them.
The third interfacing point is the biometric sensor, located in the interior of the
main handle, and haptic system, located in the main and front handles. These
two systems work as a conjunction to capture the user’s HR and HRV data and
provide a physical feedback related to it (mimicking their own heart rate, a cyclic
pattern to guide breathing, etc.) The haptic system is comprised of 4 ERM
vibration motors that can be controlled independently in pairs.
The last interface with the user will be the Therabody App. The THE DEVICE
includes a Bluetooth chipset that enables the main functionalities of the product
to be controlled via phone. Concurrently, the biometric data can be accessed by
Therabody App in order to display the information to its user and provide
recommendations based on it.
The device was designed to be a “Recovery Hub”. Based on a percussive
therapy device, the ergonomics of the triangular handle allows you to reach
virtually any part of your body, but this time with a range of therapies beyond
percussive (heat, vibration, Infrared light, cold). The LEDs providing the infrared
light therapy are built in the device and can be used in conjunction with the rest
of the therapies.
The device will also be the first percussive therapy device with a built-in
biometric sensor the reads your HR and HRV, and a haptic system (vibration
motors inside the handles) that can provide different feedback depending on the

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 4 of 24
data.
This device is contraindicated against and should not be used by those with
acute or severe cardiac, liver, or kidney disease/condition or other underlying
conditions linked to these diseases. It is not recommended that this device is
used over various skin infections and conditions, or directly over a surgical
site/hardware, bone fracture, or if you are experiencing unexplained pain in the
area. It is also advised that this device is not used for those who have
neurological and/or peripheral conditions as well as other conditions that may
influence or alter sensation and perception. In the event of any adverse reaction,
discontinue use. Please consult your physician prior to use if you are unsure if
you have any of the previously listed conditions. Immediately stop using the
device at the first sign of discomfort.
Adult supervision should be provided for those under the age of 18 using this
device. If you have any medical concerns regarding the use of this device,
please consult your physician prior to use.
The Supersoft attachment should not be used more than 2 minutes at one time
to avoid overheating and/or melting.
1.1 Contraindications
The following are circumstances where the potential risks may outweigh the
benefits. Consult a medical professional before use.
· Skin rash, open wounds, blisters, local tissue inflammation, bruises, infections
or tumors
· Deep vein thrombosis, osteomyelitis
· Bone fracture or myositis ossificans
· Hypertension (uncontrolled)
· Acute or severe cardiac, liver, or kidney disease
· Neurologic conditions resulting in loss or altered sensation
· Direct application to the face, throat, or genitalia
· Bleeding disorders
· Recent surgery or injury
· Connective tissue disorders
· Peripheral vascular insufficiency or disease
· Medications that thin the blood or alter sensations
· Direct pressure over surgical site or hardware
· Direct pressure over eyes or throat
· Extreme discomfort or pain felt by client
· Severe scoliosis or spinal deformity

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 5 of 24
· Pacemaker, ICD, or history of embolism
For a full list of contraindications and additional warnings, please visit
www.therabody.com. Patents & Pending Patents at www.therabody.com.
Used to identify symptoms or medical conditions for a person to not receive a
particular treatment or procedure because it may be harmful.
1) Never use this device over any damaged tissue or other type of skin
condition can result in delaying the best medical treatment.
2) Do not use if you have had any surgery procedure –consult your doctor.
3) Never use the device to the patient with Implant Electronic Devices (such
ascardiac pacemaker).‘
1.2 Precautions
Due care is required in these circumstances and the use of the devices may
need to be modified (such as attachment used, the force applied, the position
of the body, avoidance of use in direct contact with an area, etc.). Where
appropriate or if you have any concerns, seek the advice of a medical
professional.
• Hypertension (controlled)
• Osteopenia
• Osteoporosis
• Pregnancy
• Diabetes
• Varicose veins
• Bony prominences or regions
• Abnormal sensations (e.g., numbness)
• Sensitivity to pressure
• Recent injury or surgery
• Scoliosis or spinal deformity
• Medications that may alter client sensations

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 6 of 24
1.3 Warnings
BEFORE USING THE OR CHARGING THE Theragun PRO PLUS, READ ALL
THE INSTRUCTIONS AND CAUTIONARY MARKINGS IN THIS MANUAL, O,
AND ON Theragun PRO PLUS.
Theragun PRO PLUSis intended for commercial or home use. We strive to
make the Theragun PRO PLUS as safe as possible. This is an advanced
mechanical device with electric components. If the Theragun PRO PLUS and its
accessories are not used or maintained properly, there is a risk of fire, electric
shock, or injury. Hazard can be result from unauthorized modification of the
Theragun PRO PLUS
Do not place your finger or any objects near the metal piston above the
attachment while the Theragun PRO PLUS is in use or immediately after use.
The metal piston can become extremely hot.
Do not use any attachments other than the ones provided by Therabody. and
only as instructed by Therabody.
Do not use Theragun PRO PLUSon your head or near your genitals.
Do not use Theragun PRO PLUSabove the waist during pregnancy.
Do not get hair caught in any moving part of the Theragun PRO PLUS.
Do not use t Theragun PRO PLUS above your Adam’s Apple or C4.
Do not get Theragun PRO PLUS wet. Only clean Theragun PRO PLUS with a
magic eraser or a lightly damp towel.
Do not block the vents of the motor.
Never expose Theragun PRO PLUS to water.
For long-term storage, Theragun PRO PLUS should be fully charged.
This appliance is not intended to diagnose, treat, or prevent any diseases.
This appliance contains batteries that are non-replaceable.
When using the Theragun PRO PLUS, these basic precautions should always be
followed including:
1. USE ONLY AS INSTRUCTED. Use the Theragun PRO PLUS as described in
this Theragun PRO PLUS Instruction Manual. Use only Theragun
recommended accessories and replacement parts. Do not carry out any
maintenance other than what is shown in the Theragun PRO PLUS Instruction
Manual or advised by Theragun.

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 7 of 24
2. NOT FOR CHILDREN. The Theragun PRO PLUS are not intended for use by
young children or persons with reduced physical, sensory or reasoning
capabilities, or lack of experience and knowledge, unless they have been given
supervision or instruction by a responsible person. Do not allow the Theragun
PRO PLUS to be used as a toy. Close attention is necessary when used by or
near children. Children should be supervised to ensure that they do not play with
the Theragun PRO PLUS
3. CHARGING LOCATIONS. The Theragun PRO PLUS should be charged
indoors in a well ventilated, dry location. Do not charge Theragun PRO PLUS
outdoors, in a bathroom or within 10 feet (3.1 meters) of a bathtub, or pool. Do
not use the Theragun PRO PLUSon wet surfaces and do not expose product to
moisture, rain, or snow. Do not use Theragun PRO PLUS in the presence of
explosive atmospheres (gaseous fumes, dust, or flammable materials). Sparks
may be generated, possibly causing a fire.
7. DO NOT OVERCHARGE. Do not leave connect Theragun PRO PLUS to the
charger for more than an hour after the Theragun PRO PLUS has been fully
charged. The Theragun PRO PLUS includes a protection system to avoid the
risk of overcharging, however, overcharging the Theragun PRO PLUS may
reduce its life over time. Can use the adaptor 5v-15v ,2.5A adaptor .
8. DO NOT BLOCK MOTOR VENTS. Do not put any obstructions into vents. Do
not use if any vent is blocked. Keep free of dust, lint, hair, and anything that may
reduce airflow. Keep hair, loose clothing, and fingers from vents and moving
parts.
9. DO NOT BURN OR INCINERATE THE Theragun PRO PLUS. The internal
battery may explode, causing personal injury or damage. Toxic fumes and
materials are created when battery is burned.
10. DO NOT CRUSH, DROP, OR DAMAGE. Do not use a charger that has
received a sharp blow, been dropped, run over, or damaged in any way.
11. BATTERY CHEMICALS CAUSE SERIOUS BURNS. Never allow the
internal battery to come into contact with the skin, eyes, or mouth. If a damaged
battery leaks chemicals, use rubber or neoprene gloves to dispose of it. If skin is
exposed to battery fluids, wash with soap and water and rinse with vinegar. If
eyes are exposed to battery chemicals, immediately flush with water for 20
minutes and seek medical attention. Remove and dispose of contaminated
clothing.
12. DO NOT OPERATE UNDER BLANKET AND PILLOW. Excessive heating
can occur and cause fire, electric shock, or injury to persons.
13. STORING THE Theragun PRO PLUS Store in a cool, dry place. Do not
store the Theragun PRO PLUS where temperatures may exceed 50°C/104°F

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 8 of 24
such as in direct sunlight, in a vehicle, or metal building during the summer.
14. DISPOSING OF BATTERIES. The internal Theragun Lithium-ion batteries
for the Theragun PRO PLUS are more environmentally friendly than some other
types of batteries (e.g., nickel-cadmium). Always dispose of the Theragun PRO
PLUS according to federal, state, and local regulations. Contact a recycling
agency in your area for recycling locations. Even discharged batteries contain
some energy. Before disposing, use electrical tape to cover the Theragun PRO
PLUS’s 12V connecter to prevent the battery from shorting, which could cause a
fire or explosion.
15. DO NOT DISASSEMBLE. Disassembly or incorrect reassembly may result
in the risk of electric shock, fire, or exposure to battery chemicals. The warranty
will be void if the Theragun PRO PLUS are disassembled or if any parts have
been removed.
16. SERVICE. If the Theragun PRO PLUS is not working properly, has received
a sharp blow, has been dropped, damaged, left outdoors, or dropped into water,
do not use and contact Theragun by emailing info@therabody.com for repair or
service. Do not attempt to repair or disassemble the Theragun Theragun PRO
PLUS which may result in an electric shock or fire.
17. DO NOT USE WHILE BATHING OR IN THE SHOWER, TUB, OR SINK. Do
not place or store the Theragun PRO PLUS where it can fall or be pulled into a
tub or sink. Do not place in or drop into water or other liquid. Do not reach for an
appliance that has fallen into water. Unplug immediately.
18. THERMAL LIMITER. The Theragun PRO PLUS employs an automatic reset
thermal limiter that shuts off the Theragun PRO PLUS to prevent overheating and
fire.
Changes or modifications not expressly approved by the party responsible for
compliance could void the user’s authority to operate the equipment.
Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Strangulation due to cables and hoses particularly due to excessive length
Potential allergic reactions to accessible materials used in the ME
EQUIPMENT
Available for immediate use when device cool from the maximun storage
temperature between uses and Device warm from the minimum storage
temperature between uses.
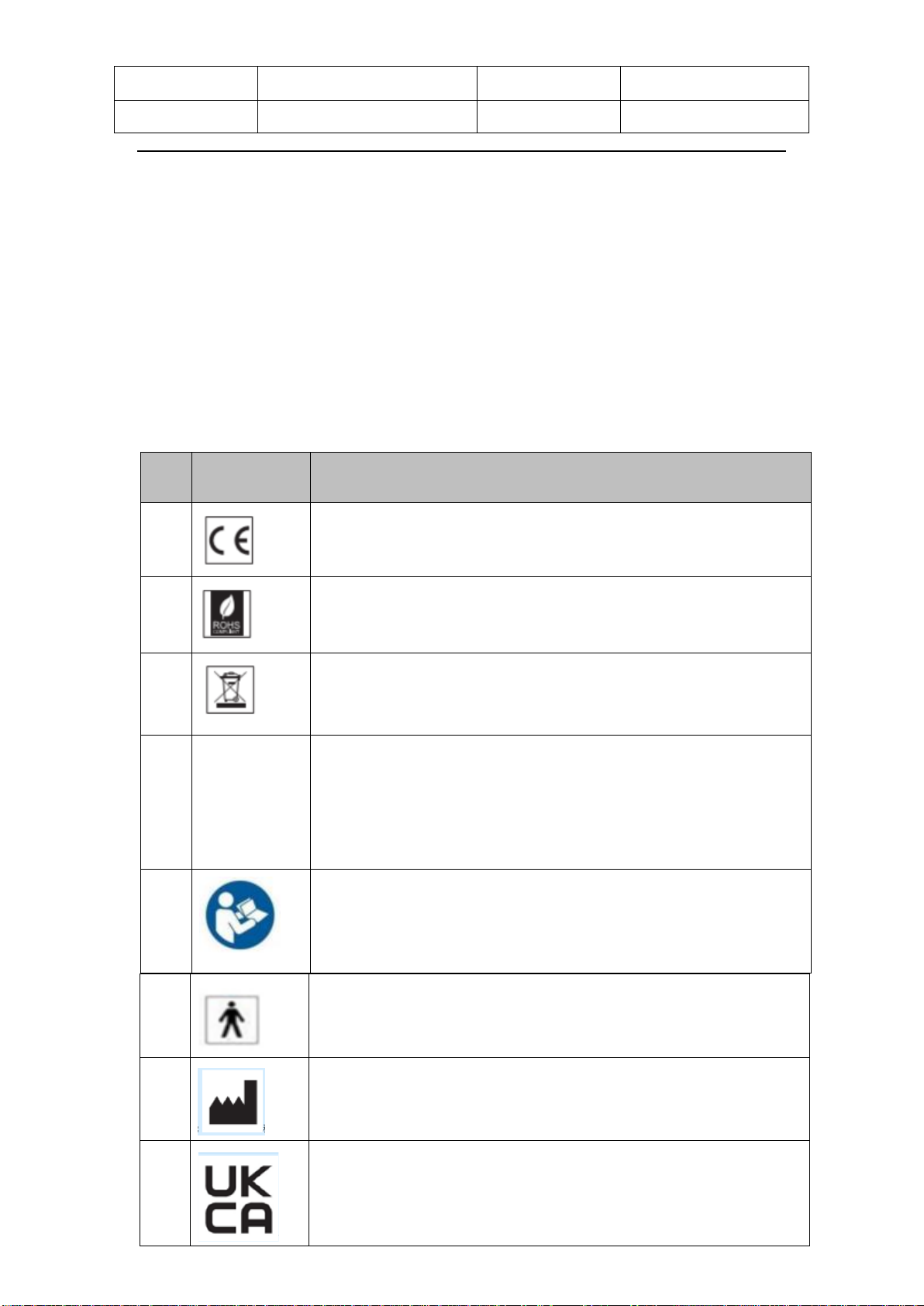
1.4 Label & Symbols
The following labels and symbols appear on Theragun Theragun PRO PLUS:
No. Symbols Description
1
CE mark
2 the Restriction of Hazardous Substances
3
“WEEE (Waste Electrical and Electronic Equipment)”.The
waste products should be handled legally.
4 IP22
IP Classification: The first number 2: Protected against
access to hazardous parts with a finger, and the jointed test
finger of 12 mm Ф, 80 mm length, shall have adequate
clearance from hazardous parts, and protectedagainst solid
foreign objects of 12.5 mm Фand greater.
5
Please read the user manual before use.
6
Type BF apply part
7
Manufacturer information
8
UKCA Mark for UK
Page 9 of 24
Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 10 of 24
2. Intended Use & Indications for Use
Provide a guided and non-guided in-dept therapy around the body with different
therapies: relaxation therapy through physical feedback, cold therapy, heat
therapy, vibration therapy, infrared light therapy, and percussive therapy.
Depending on the protocol, use cases will be different but the main ones are: to
increase blood blow, to reduce inflammation (specific to cold), reduce tension
and knots, reduce soreness, reduce panic, and increase relaxation.
-A second type of use case is related to the PPG sensor, which allows F1 to
measure and process the user’s HR and HRV. This data can be use for
informative purposes, but also to guide certain settings of the treatment while it
happens, in order to improve its efficacy.
3. Device Description
Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 11 of 24
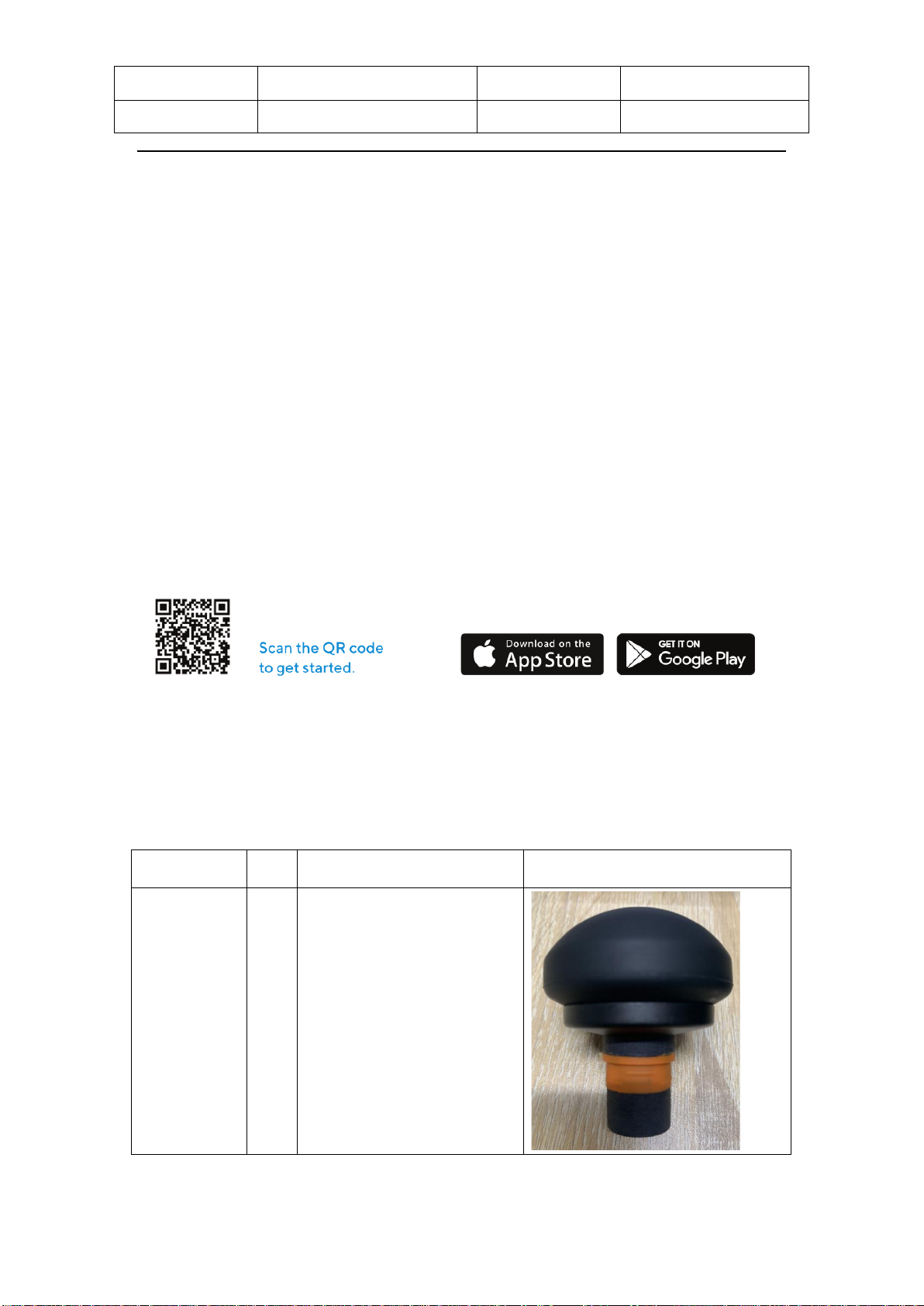
A. Button pad
B. LCD screen
C. Heart rate sensor
D. Attachment connector
E. LED treatment module
F. Type-C Port
3.1 Smart Features
Theragun device is smart device enabled with Bluetooth connectivity, designed
to pair seamlessly with the Therabody app. When you download the app, you’ll
be guided through a step-by-step setup and shown how to pair your device.
Once paired with your device, the app can remotely adjust speed, activate guided
treatment presets, and make real-time recommendations based on your feedback
and recent activity. It’s truly your intuitive wellness partner on your journey to
feeling better and moving more freely. Every day.
3.2 Attachments
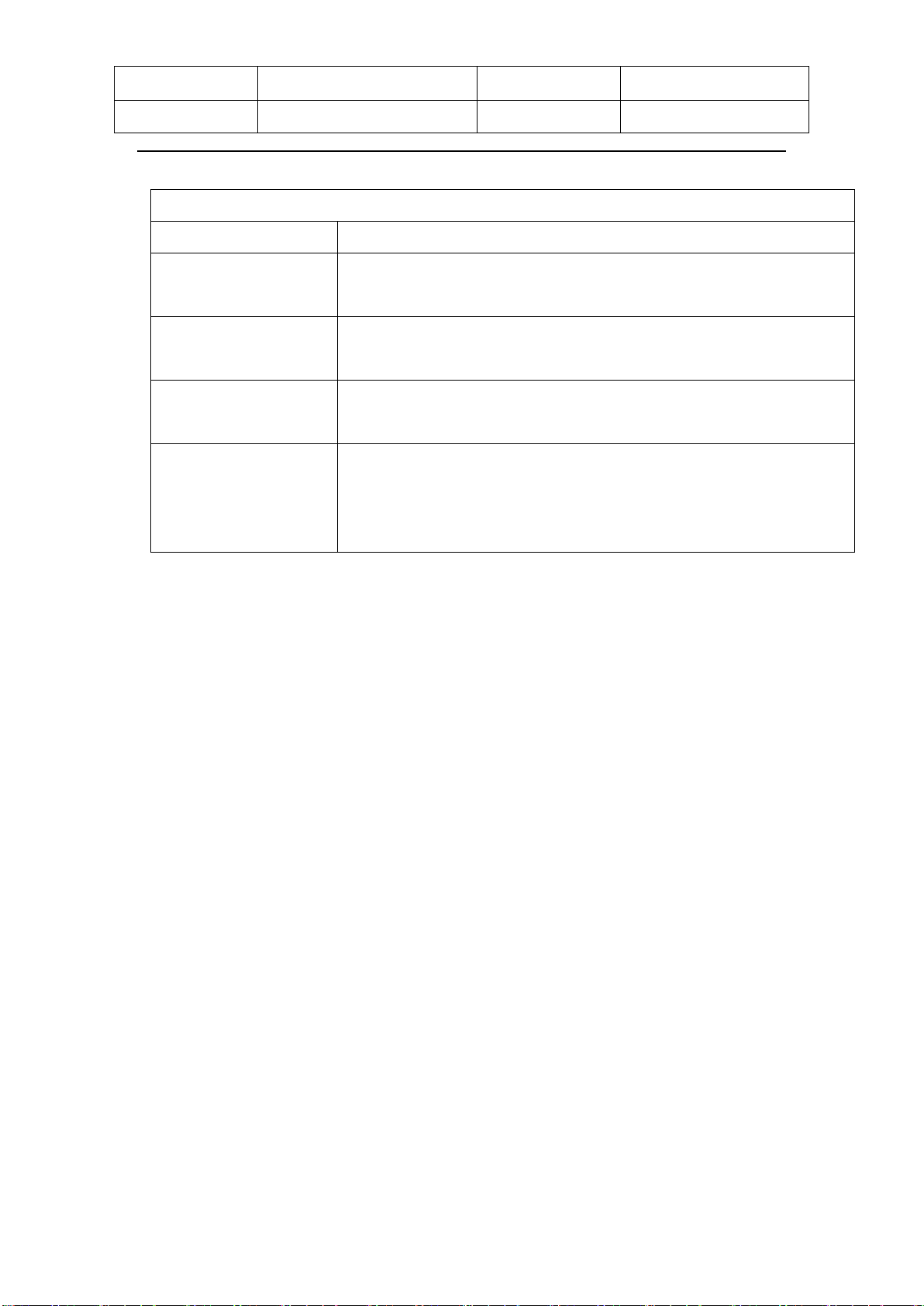
Theragun device comes with 4 different functional attachments designed for
every muscle and need. Applied parts is massager head
Attachments QTY Using Figure
Heating
attachment 1
For use on tender areas or
near bones.This attachment
has a heating function, so that
you can quickly feel the effects
of heat treatment.

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 12 of 24
Cold
attachment 1
For use on tender areas or
near bones.This attachment
has a cold function, so that
you can quickly feel the effects
of cold treatment.
Vibration
attachment 1
For use on tender areas or
near bones. This attachment
has a vibration function, so
that you can quickly feel the
effects of vibration treatment.
Dummy
attachment 1 For use on tender areas or
near bones
3.3 Accessories
A type-c (USB-C to USB-C PRO PLUS USB-A or Use C to C)Line is provided
together with the Theragun device.
3.4 Getting started
Step 1 Turn on and float
Firmly press and hold the center button on the button pad, then float the Theragun
device across areas in need. Use the up (Λ) and down (v) buttons on the button
pad to adjust speed. Apply to moderate pressure as needed.
Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 13 of 24
Step 2 Get the Therabody app
We’ll guide you through a stress-free setup and show you how to get the most out
of your fully personalized app experience.
Step 3 Feel the difference.™ Every day.
Take a deep breath and treat yourself with guided treatment presets or go
freestyle with 5 preset speeds. It’s all designed for you.
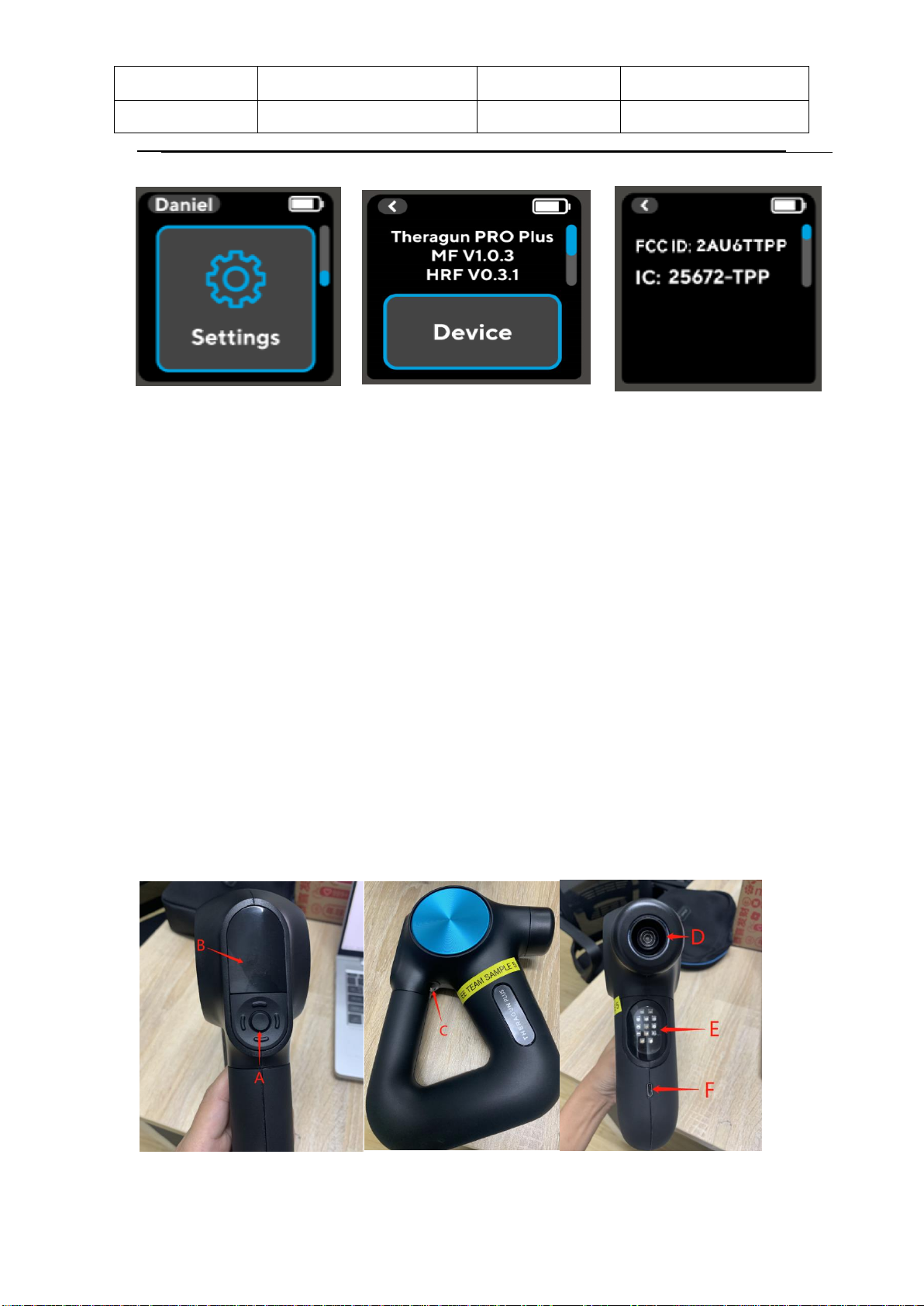
Set up :Select the mode and press the center button to enter the "Quick start"
mode. The number in the upper left of the screen shows the treatment time, the battery
power in the upper right corner, the current set speed in the middle, and the small icon
in the lower part is from left to right: Play/pause icon -> percusive icon(this icon is not
displayed if the device is connected to a vibration or cold attachment, because Vibration
and Cold attachment disable the percusive function) -> functional attachment icon(if
the device is connected to a function attachment, the corresponding attachment icon is
displayed here) -> LED icon.
HR sensor: Double click the heart rate sensor with your finger to enter the heart
rate detection interface, accompanied by the release of the vibration motor. Do
not move your finger on the heart rate detection site. After the heart rate is
measured and displayed on the screen. Double-click the heart rate detection
and return to the main interface or press the center button to return to the main
interface (the heart rate graph is displayed at the top of the heart rate detection
interface, HR value is displayed in the middle ). Holding down the middle button
will turn off the device at any time
3.5 Using the Theragun device
1) To turn on the Theragun device, firmly press and hold the center button on
the button pad .
2) Press the up (Λ) and down (v) arrows to toggle between 5 preset speeds
and adjust your treatment level.
3) To turn off the Theragun device, firmly press and hold the center button on

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 14 of 24
the button pad.
3.6 Connecting the attachments
Please follow the instructions in the below picture. Align the functional attachment
with the attachment connection of the device, press into it and turn clockwise until
the attachment is completely locked with the device.
3.7 Charging the Theragun device
1) Your device includes a USB-C to USB-C PRO PLUS USB-A cable so you
can use a wider variety of adapters. The Battery capacity will show on the LCD
screen.
2) Charging is complete when LCD screen show you 100% with the battery

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 15 of 24
icon.
3.8 Discharging personal device from Theragun device
1) You can use the Theragun device to charge your personal device by
connecting one end of the USB-C cable to the Thregun's Type c port, and then
connecting the other end of the cable to the port that fits your personal device.
Then press center button,you'll see a picture of the discharge on the LCD
screen.
3.9 LED module function use
You can Press the center button to turn on the device,an then select “Quick start”
mode.Then you can see LED is ON. If you don`t want motor running,you can use the “v”
key to down the motor speed to 0. If you have connect attachment on device,if you
don`t want to enable the attachment function,you can use“v”to down it to level 0 to
disable it too,or you can just take it off the device.

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 16 of 24
4. Cleaning Instructions
For the most hygienic Theragun device experience, sanitize it after each use by:
1) Wiping the device with a disinfectant wipe to remove any residue.
2) Once the device is residue-free, use a new wipe to disinfect the surface
area by wiping all sides in a downward motion.
5. Trouble Shooting
In the event that the device fails to perform as intended, the following
notes will help to identify potential problems with the device and its setup.
Problem Solution
Device turns off automatically low powerMaybe reach the set time or
Device cannot turn on Maybe battery is in low power and you
should recharge batteries. .
treatment or-Device shuts off in mid
only after few treatments Check and recharge batteries.
Cannot charge battery Check if the adapter output connector
insert into the charging port in the
device firmly
6. Service
Operation Instruction has no parts you can fix. Do not try to repair it. If
servicing is required, please contact the selling retailer. All returned units to
the manufacturer for repair, including Warranty repair and Out-Of-Warranty
repair, must include the following:

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 17 of 24
During Warranty Period with proof of Purchase (store receipt).
RMA Number: Should your product become defective during the warranty
period, call our service center or contact your retailer.
Package the item securely and return it prepaid/insured – along with
proof of purchase to:
Therabody, Inc.
6100 Wilshire Blvd. Suite 200 Los Angeles, CA 90048-5107, USA
To ensure prompt repair, provide complete, legible name, address and
phone number information, RMA number and a note indicating the nature
of the product defect and a copy of the original invoice issued for purchase
of the unit. We will Repair or Replace (at our sole discretion) product at no
charge. Ship unit to the manufacturer in the original container with all
accessories and information as required above.
Service Life
When used and maintained as instructed, the expected service life of Theragun
Theragun PRO PLUS two years.
7. Product Specification
Basic Unit Characteristics
Indicator Light No
LCD screen Yes
Housing Materials PC
Additional Features
Environment for
operation
Temperature: 0 ~ 40° C
Relative humidity: <93% RH
Environment for
storage
Temperature: -10° C ~ 50° C
Relative humidity: 10~95% RH
Use atmospheric
pressure 70-106Kpa
Software version MF=V0.29
Heat attachment level Level1=40℃;Level2=48℃;Level3=55℃.
Cold attachment level Level1=15℃;Level2=10℃;Level3=5℃.
Vibration attachment Level1=2600rpm;Level2=2900rpm;Level3=3200rpm.

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 18 of 24
level
LED module Wavelength: IR=840~860nm; RED=650~670nm; Level=ON/OFF.
8. Safety, EMC
This device is Class II equipment with type BF applied. It complies with
Medical Electrical Safety Standards (IEC 60601-1).
This device also complies with Medical EMC Standard (IEC 60601-1-2).
The has been tested and found to comply with the electromagnetic compatibility
(EMC) limits for medical devices to IEC 60601-1-2: 2007. These limits are
designed to provide reasonable protection against harmful interference in a
typical medical installation.
CAUTION:
Do not apply the device near any devices with Electromagnetic Interference
(EMI), such as cell phones, Magnetic Resonance Imaging (MRI), computerized
axial tomography (CT), diathermy, Radio Frequency Identification (RFID), etc.
or MR environment. EMI, RF devices or MR environment may affect the normal
function of the device or would cause user injury.
The patient is an intended operator. The ME EQUIPMENT shall not be
serviced or maintained while in use with a patient.
Manufacturer’s declaration – electromagnetic immunity
1* WARNING: Use of this equipment adjacent to or stacked with other
equipment should be avoided because it could result in improper operation. If
such use is necessary, this equipment and the other equipment should be
observed to verify that they are operating normally.”
2* WARNING: Use of accessories, transducers and cables other than those
specified or provided by the manufacturer of this equipment could result in
increased electromagnetic emissions or decreased electromagnetic immunity
of this equipment and result in improper operation.”
3* WARNING: Portable RF communications equipment (including peripherals
such as antenna cables and external antennas) should be used no closer
than 30 cm (12 inches) to any part of the ME EQUIPMENT, including cables
specified by the manufacturer. Otherwise, degradation of the performance of
this equipment could result.”

Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 19 of 24
Any Changes or modifications not expressly approved by the party responsible for compliance
could void the user's authority to operate the equipment.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) This device may not cause harmful interference, and (2) this device must accept
any interference received, including interference that may cause undesired operation.
Note: This equipment has been tested and found to comply with the limits for a Class B digital
device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential installation. This equipment generates,
uses and can radiate radio frequency energy and, if not installed and used in accordance with the
instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does
cause harmful interference to radio or television reception, which can be determined by turning
the equipment off and on, the user is encouraged to try to correct the interference by one or
more of the following measures:
—Reorient or relocate the receiving antenna.
—Increase the separation between the equipment and receiver.
—Connect the equipment into an outlet on a circuit different from that to which the receiver is
connected.
—Consult the dealer or an experienced radio/TV technician for help.
This device complies with Innovation, Science, and Economic Development Canad licence-exempt
RSS standard(s). Operation is subject to the following two conditions:
(1) this device may not cause interference, and
(2) this device must accept any interference, including interference that may cause undesired
operation of the device.
Le présent appareil est conforme aux CNR d' Innovation, Sciences et Développement économique
Canada applicables aux appareils radio exempts de licence. L'exploitation est autorisée aux deux
conditions suivantes :
(1) l'appareil nedoit pas produire de brouillage, et
(2) l'utilisateur de l'appareil doit accepter tout brouillage radioélectrique subi, même si le
brouillage est susceptible d'en compromettre le fonctionnement.
This device complies with FCC/ISEDC radiation exposure requirement set forth for an
uncontrolled environment. The transmitter must not be co-located or operating in conjunction
with any other antenna or transmitter.
L'appareil est conforme aux exigences d'exposition aux rayonnements imposées par la FCC/ISEDC
pour les environnements non contrôlés. L'émetteur ne doit pas coexister ou fonctionner en
synergie avec une autre antenne ou un autre émetteur.
Document Name User Manual Document No. TG-TPP-001
Model Theragun PRO PLUS Rev 01
Page 20 of 24
Table 1
declaration - electromagnetic emission
Emissions test Compliance
RF emissions
CISPR 11
Group 1
RF emissions
CISPR 11
Class B
Harmonic emissions
IEC 61000-3-2 Not applicable
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Not applicable
Table of contents
Other THERAGUN Massager manuals

THERAGUN
THERAGUN Halo User manual

THERAGUN
THERAGUN G2PRO User manual

THERAGUN
THERAGUN RecoveryAir User manual

THERAGUN
THERAGUN Prime User manual

THERAGUN
THERAGUN Elite User manual

THERAGUN
THERAGUN Elite User manual

THERAGUN
THERAGUN Wave Duo User manual

THERAGUN
THERAGUN Elite User manual

THERAGUN
THERAGUN LIV User manual

THERAGUN
THERAGUN mini User manual