ThermoTek Artek PRO User manual

Cold, Heat, and Compression
Therapy Unit
INSTRUCTIONS FOR USE
0P1DATP01M-EN Rev X3
Need Help?
See our instructional videos
on how to set up and use
your ARTEK Pro

• This device is intended for
single-patient use only. Secondary use
can cause risk of infection or injury.
• Do not apply wrap directly against
bare skin. Always use a skin barrier or
a thin piece of clothing between skin
and wrap.
• The skin barrier must be kept dry. If
barrier develops moisture, stop
therapy and change barrier.
• Be sure to connect wrap and hose
with audible click. Failure to connect
system may cause system to leak.
• Only use the ARTEK Pro system with the
power cord and accessories provided
with the device. The use of other
accessories may result in serious injury.
• Do not use in a sterile environment or
place wraps directly over an open
wound or breached skin.
Attention: The potential for cold injury is greater with extremity application.
With foot/ankle or hand/wrist application, be sure to perform frequent
checks of the toes or fingers for discoloration or numbness. If changes are
observed, discontinue use immediately and consult your physician.
1
The ARTEK Pro is indicated to provide
localized thermal therapy for post-
traumatic and post-surgical conditions
and aid in the reduction of edema
associated with soft tissue injuries,
post-operative edema, and ligament
sprains.
Indications for Use
DO NOT use the ARTEK Pro on patients
experiencing any of the following:
• Raynaud’s phenomenon
• Vasospastic conditions
• Cold allergy or hypersensitivity
• Cryoglobulinemia
• Cold agglutinin disorders
• History of cold injury
• Limited mobility
• Reduced perception of pain
• Medication affecting sensation
Contraindications
Special care and attention should be
made when prescribing the ARTEK Pro
for the following patients diagnosed
with the following:
• Arthritis
• Peripheral vascular disease
• Compromised circulation
• Children under the age of 12
• Decreased skin sensitivity
• Hypercoagulation disorders
• Diabetes
Precautions
Warnings
Cold Injury Warning
Prolonged exposure to cold has the
potential to cause injury to tissue. There
is a potential for cold tissue injury even
when providing cold therapy within your
prescribed treatment. If unusual
swelling, irritation, skin discoloration or
discomfort occurs, immediately
discontinue use of the ARTEK Pro and
consult your health care professional.
• Do not attempt to service this device.
Attempting to do so may lead to
electric shock.
• Do not use in a sterile environment or
place wraps directly over an open
wound or breached skin.
• Do not drop the device or use if system
is physically damaged. Keep
wrap/hose away from sharp objects.
• Do not place therapy hose or power
cord near/around your neck;
strangulation risk.
• Do not cast over the ARTEK Pro therapy
wraps.
• Do not spill water over the top of the
device. Dry any excess water on the
outside of the device prior to use.
• Use only water in the system.
• Do not smoke near system or use
system near open flame.

ARTEK Pro provides cold, heat, and/or intermittent compression therapy to a
localized area by flowing temperature-controlled water and/or air through an
applied therapy wrap. The intended user is a licensed medical professional, a
patient, or a caregiver. The user should be able to read and understand directions,
warnings, and cautions provided in these user instructions. The user should be
physically capable of performing all the instructions provided in these user
instructions.
Operating Principle
• Read and understand all warnings and instructions for use before using the
device.
• The ARTEK Pro is prescription use only. Do not use without a prescription.
•
It is the responsibility of the prescribing physician to define the therapy use
parameters for each patient. If you are a patient, and are unclear as to the
details of your prescribed therapy, contact your prescribing physician. This
single-patient use system, and its components, are non-sterile and are for
prescription use only.
Before using the ARTEK Pro
Understanding your Prescription
The ARTEK Pro can administer cold, heat and/or intermittent compression.
Only use the therapies that are prescribed.
If cold or heat therapy is prescribed, your prescription will include the
prescribed treatment ON time, either 30 or 60 min.
If your prescribed therapy is ‘as tolerated,’ you should choose therapy ON
time that works best for you.
Thermal therapy on the ARTEK Pro rests for 15 min and is not adjustable by
the user. It is important for your safety that you do not skip the rest period.
If you have any questions regarding the details of your prescription,
contact your physician.
2

Universal Therapy Wrap Kit
• Includes 1 wrap, 4 straps, 2
connection tabs, and wrap
instructions for use
• Includes skin barrier for safe
application
• Wrap is applied to site of injury to
apply therapy
Power Supply and Cord
• Provides wall power to device
• Only use the manufacturer- supplied
power supply cord with this system
The ARTEK Pro Therapy System is a single-patient use system, designed to deliver
effective and safe cold, heat, and/or compression therapy. These user instructions
describe the operating and safety procedures for the ARTEK Pro. Read these user
instructions in their entirety, prior to using the ARTEK Pro.
Unpacking the System
The ARTEK Pro system is provided with the following components. If the packaging is
damaged, or any listed pieces are missing, please contact customer service at
866.280.5844.
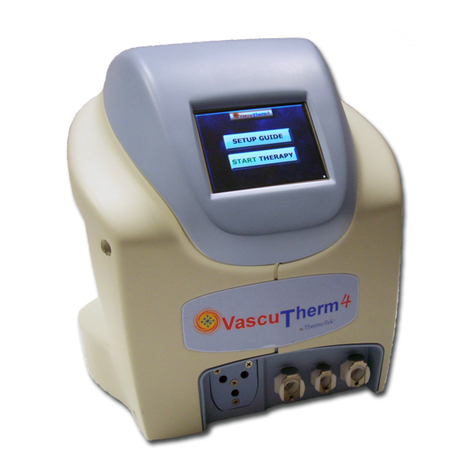
ARTEK Pro Device
• Keypad: Allows user to select
therapy options and displays
therapy state
• Hose: Connects the device to the
therapy wrap
• Hose Connectors: Located at the
end of the hose and used to
connect the wrap to the device
• Reservoir/Cap: Remove the cap to
put water in the device
• Overflow Basin: Area around the
reservoir to catch any overflow
water spills
• Power Input: Located on the back
of the device and connects wall
power to device
• Air Vents: Allow airflow to and
through the internal system
components
Keypad
Reservoir/Cap
Air Vents Hose
Hose Connectors
Power Input
(on back of the device)
Overflow Basin
3
ARTEK Pro System

Power Indicator: The power indicator will illuminate green when the
system is supplied with wall power. To turn the device off, disconnect
from wall power.
Fault Indicator: The fault indicator illuminates yellow when there is a
fault or issue with the system. Other yellow indicators may
illuminate, depending on the nature of the fault. Refer to the
Troubleshooting section for more information about the fault.
Time Button/Indicator: The time button changes the active therapy
run time between 30 and 60 minutes. This button is disabled once a
therapy session is started.
Cold Therapy Button/Indicator: This button starts and stops delivery of
cold therapy. The indicator above the button illuminates blue when
therapy is active and blinks blue when the therapy is in rest mode. If the
light illuminates or blinks yellow, this indicates a fault. Refer to the
Troubleshooting section for more information about the fault.
The cold button is disabled while heat therapy is active.
Heat Therapy Button/Indicator: This button starts and stops delivery of
heat therapy. The indicator above the button illuminates blue when
therapy is active and blinks blue when the therapy is in rest mode. If the
light illuminates or blinks yellow, this indicates a fault. Refer to the
Troubleshooting section for more information about the fault.
The heat button is disabled while cold therapy is active.
Compression Therapy Button/Indicator: This button starts and stops
delivery of compression therapy. The indicator above the button
illuminates blue when therapy is active. If the light illuminates or blinks
yellow, this indicates a fault. Refer to the Troubleshooting section for
more information about the fault.
Compression therapy can be used in combination with cold or
heat therapy or as a stand-alone therapy.
4
The ARTEK Pro device is controlled using a simple, touchpad operation. The
touchpad provides the following information and control. Refer to this section
during set-up and therapy instruction.
ARTEK Pro Keyboard

4. Remove the
reservoir cap and
fill the reservoir
with water. The
water may drain
into the system.
Press the heat
button to start
water flow into
the system and
wrap.
5. Continue filling
the reservoir until
the water volume
remains stable.
General Cleaning
The device can be wiped down with a
damp rag or mild disinfectant cleaner.
The wrap can be hand washed with
mild soap and water. Dry fully before
using for therapy. DO NOT use the
wrap if it is wet.
System Storage
1. Unplug the power cord from the
electrical outlet and disconnect from
the back of the device.
2. Disconnect the wrap from the device
hose.
3. Remove the reservoir lid and empty
the system. Leave lid off of reservoir
when storing the system.
4. The wrap cannot be drained, however
the fluid will evaporate over time.
5. The system can be stored open on a
shelf or in a breathable container. DO
NOT store in a closed container or in a
plastic bag. This may cause mold to
form inside the unit.
TIP: Do not open the reservoir lid
when the system is off/not running
with the wrap connected. Doing so
may cause the fluid to overflow out
of the reservoir into the overflow
basin.
See our instructional videos
on how to set up your
ARTEK Pro.
The ARTEK Pro is now ready to use.
5
Cleaning and Maintenance
Setting up your ARTEK Pro
1. Remove all system components
from the packaging. To prevent
microbial growth, DO NOT store the
system, or its components, in the
plastic bags/packaging after using
the system.
2. To set up the ARTEK Pro, you need to
fill the wrap with water. Have 2 cups
of water in an easy to pour from
container. Connect the wrap to the
hose by connecting all 3 hoses. You
will hear an audible click when
connected correctly.
3. Connect the system
to wall power by
connecting the
power cord and
power supply to the
power inlet on the
back of the unit and
to a wall outlet.
7. Top off water in
reservoir until it is 1”
from the top. Replace
the reservoir cap and
press the heat button
again to stop the
system.
6. Pick up the wrap by
the hose and shake it
to remove any major
bubbles. Smaller
bubbles will
evacuate over time.
TIP: If system is to be stored for an
extended period of time, isopropyl
alcohol can be added to the
reservoir. Let the system run for 10
min and then drain as described
above. This can help prevent
biogrowth in the system.

6
The ARTEK Pro system can deliver cold or heat thermal therapy that can be
combined with compression therapy. Refer to your physician’s prescription on
which therapy you should use and their instructions on the duration and frequency
of use.
Using the ARTEK Pro
Apply the wrap to the site of injury. For instructions, see the
instructions provided in the wrap kit or see our Universal Wrap
instructional videos
Thermal Therapy
1. With the wrap applied, set the active
therapy time using the time
button. The LED will indicate the
active therapy time selected.
2. To start thermal therapy, press either
the cold or heat button. The
blue LED will illuminate above the
button to indicate the therapy has
started. If you need to stop therapy,
press the therapy button again.
3. During cold therapy, the water will
super cool for 40 seconds prior to
initiating flow. The system will start
pumping temperature-controlled
water to the wrap.
4. While administering Cold therapy,
you may feel changes in water flow
to the wrap. This is normal.
Attention: The rest period is
important for the safety of
therapy. Do not skip the rest
period.
1. To start compression therapy, press
the compression button. The blue
LED will illuminate above the
compression button.
5. After the set therapy time is
complete, the thermal therapy will
transition into rest mode and the
indicator light will start to blink. After
the 15 min of prescribed rest, the
cold therapy will restart.
Cold or Heat therapy cannot be
restarted until the 15 min rest time is
completed.
TIP:
Be sure to stop compression therapy prior to removing the wrap.
Inflating the wrap while not applied could cause damage to the wrap.
2. Compression will deliver to the site of
injury until therapy is stopped by
pressing the compression button
again. Compression does not enter
rest periods like thermal therapy.
Wraps can be applied as anatomy requires, as long as 3 rules are followed:
1. Always use a dry skin barrier between your skin and the wrap.
2. Apply the wrap and tubing flat without any kinks or folds.
3. Do not apply the wrap too tightly.
Compression therapy can be delivered alongside thermal therapy or as a stand
alone treatment.
Compression Therapy

•
Terminate therapy immediately and remove the wrap.
•
Contact your physician.
•
Do not reinitiate therapy without first contacting your physician.
Therapy skin area is discolored, tingly, numb, blistered or is otherwise physically
altered.
Device will not turn on or power up.
•
Check power at the wall. Try new wall connection.
•
Disconnect and reconnect power cord at device inlet.
Display does not illuminate when device is powered or LEDs do not illuminate
correctly.
•
Disconnect and reconnect power to the device.
•
All lights will illuminate briefly when power is supplied to device.
•
Check power at the wall connection if no lights illuminate.
Cold or Heat button does not function.
•
Check for power and keypad issues as above.
•
The Cold and Heat buttons are disabled when the alternate therapy is active.
Stop the active therapy and retry.
Time button does not function or change treatment time.
•
Check for power and keypad issues as above.
•
The Time button is disabled when thermal therapy is active. Stop the active
therapy and retry.
Water flow to the wrap starts and stops during active cold therapy.
•
During cold therapy, you may feel burst of cold. This is normal.
•
Bursts of cold are due to fluctuation in water flow every ~5 min.
•
If changes in flow create a repeated pulsing feeling near the wrap tubing, this
can indicate fluid flow issues. See below for troubleshooting tips.
Need Help?
See our instructional videos
on how to set up and use
your ARTEK Pro. Wrap
Application
Device Set up
and Use
7
Troubleshooting
The therapy light is blinking when I start therapy.
•
Thermal therapy cycles between active and rest states.
•
The rest period is 15 min and is not adjustable by the user.
•
If the thermal therapy was stopped during the rest period, therapy will restart
in rest, indicated by the blinking therapy LED.
•
The rest period is important for safety: Do not skip the rest period.

Water is not flowing to the wrap or the wrap is not cooling or heating.
• Confirm therapy is in active mode and not in rest mode by observing that the
therapy indicator is NOT blinking. If in active mode, be sure the issue is not the
therapy modulation state. If in modulation state, flow should restart in 1 min.
• Disconnect and reconnect wrap connections.
•
When troubleshooting water flow issues, remove the wrap and place open on a
flat surface. If flow issues resolve after doing this, reapply wrap being sure the
wrap is applied with no kinks or folds and the wrap is not applied too tightly.
•
Restart therapy and confirm that the pump starts by listening for the hum and
observing agitation of fluid in the reservoir.
•
If water is flowing but flow seems slow, open and close the reservoir lid while
therapy is active. Sometimes a vacuum can form in the reservoir.
My device, wrap, or hose is leaking.
•
If the leak is coming from the wrap/hose connections, disconnect, check that the
black o-rings on the wrap connectors are in place, and reconnect to the hose. A
good connection will result in an audible click.
•
If the leak is coming from the wrap or hose, check for damage/cuts in the
material.
•
Leaks from the device may come from reservoir overflow. Remove all water
from the outside of the device and retry.
8
My device is presenting a fault - yellow lights appear on the keyboard.
•
See the next section to identify the reason for the fault and troubleshooting tips.
•
Use troubleshooting tips from this section to help resolve the fault.
Troubleshooting
Disposal Information
Do not dispose of the device or its accessories in household waste.
All therapy wraps are for single patient use only. Discard the therapy wraps per
local government / city laws on acceptable method of disposal.
For further information about your ARTEK Pro,
visit us at www.directekusa.com/artek-pro or
call us at 866.280.5844

3 3 3 3
9
Fault Identification
Fault Cause Troubleshooting
High
ambient
temp
The room
temperature is
warmer than
optimal to deliver
cold therapy or unit
is near a heat
source.
Reduce room temperature
to less than 79F and be
sure the unit is not sitting
in direct sunlight or near a
heater.
High
internal
system
temp
The system detects
higher than
expected internal
temperature.
Confirm that the airflow
vents are not blocked and
have 12" of clearance.
Be sure ambient
temperature is lower than
79F and the unit or wrap is
not sitting in direct sunlight
or near a heater.
Check that fans on both
sides are operating.
Sensor
error One or more of the
internal sensors has
failed.
Disconnect from wall
power for 10 min.
Reconnect and restart.
Software
reset
(WDT)
The software
experienced a
failure and reset.
Software error. Disconnect
and reconnect to wall
power and restart.
General System Faults
If your ARTEK Pro detects an issue, the Fault light will illuminate or blink yellow.
Other yellow lights may also appear on your keypad, indicating the nature of the
issue.
The tables below outline the faults that may present on the ARTEK Pro and the
troubleshooting tips recommended to fix the issue.
The Key below defines the different therapy LEDs that can illuminate yellow when a
fault is present. The status of the illuminated LED (on, off, blinking) is also defined for
each fault.
Key
3
Fault LED
Cold LED
Heat LED
Compression LED
On
Off
Blinking
Multi-blink
(may be 2 or 3)
If any fault continues to
present after performing
troubleshooting measures,
call Customer Service.

2
2
10
Fault Identification
Thermal Therapy Faults
Fault Cause Troubleshooting
Cold
temp
limit
The system detects
water temperature
lower than
expected.
Disconnect from wall
power for 10 min.
Reconnect and restart.
Disconnect and reconnect
the wrap to the hose and
confirm a good
connection.
Reapply wrap assuring
that there are no kinks or
folds and retry.
System
not
cooling
The system is
unable to deliver
cold therapy to the
intended
temperature.
Be sure ambient
temperature is lower than
80F and the unit or wrap is
not sitting in direct sunlight
or near a heater.
Confirm that the airflow
vents are not blocked and
have 12" of clearance.
Cold
temp
limit
(HW)
Backup temperature
sensor detects
water temperature
lower than
expected.
Disconnect from wall
power for 10 min.
Reconnect and restart.
Heat
temp
limit
The system detects
water temperature
higher than
expected.
Disconnect from wall
power for 10 min.
Reconnect and restart.
System
not
heating
The system is
unable to deliver
heat therapy to the
intended
temperature.
Be sure ambient
temperature is above 41F.
Heat
temp
limit
(HW)
Backup temperature
sensor detects
water temperature
higher than
expected.
Disconnect from wall
power for 10 min.
Reconnect and restart.

11
Fault Identification
2
3
Compression Therapy Faults
Fault Cause Troubleshooting
High
pressure
limit
The system detects
wrap pressure
higher than
expected.
Reapply wrap assuring
that there are no kinks or
folds and retry.
Avoid any external
pressure on the wrap
(squeezing or pressing on
the wrap) while in use.
Wrap
not
inflating
The wrap is not
inflating to the
expected pressure.
Check the wrap for leaks
or tears. Disconnect and
reconnect the wrap to the
hose and confirm a good
connection.
Wrap
not
venting
The wrap cannot
release the inflated
pressure.
Check the wrap for kinks or
folds.
Remove the wrap and
reapply, assuring wrap is
applied flat.
Inflation
blocked The system cannot
inflate the wrap due
to a block in the air
line.
Disconnect and reconnect
wrap to hose to confirm
secure connection.

The ARTEK Pro system (device and accessories) comes with a 90-day
manufacturer’s warranty.
Technical Information
12
ARTEK Pro Conformance Information
Quality Assurance FDA 21 CFR 820 QSR,
ISO 13485
Safety IEC 60601-1, IEC
60601-1-11
Electromagnetic
Compatibility (EMC) IEC 60601-1-2
ARTEK Pro Classification Information
US FDA Medical Device 21 CFR 878.4360
Protection Against
Electric Shock Hazard Class II per
UL/EN/IEC 60601-1
Applied Protection
Against Fluid Ingress IP21
Applied Part Type BF
Guidance and Manufacture Declaration
– Electromagnetic Emissions
Emission Test Compliance Electromagnetic Environment -
Guidance
R
adiated Emissions EN 55011
C
omplies to Group 1,
C
lass B
T
he equipment uses RF energy only
f
or its internal function. Therefore,
i
ts RF emissions are very low and
a
re not likely to cause interference
t
o nearby electronic equipment.
T
he equipment is suitable for use in
a
ll hospitals, health service clinics
a
nd homes connected to the
p
ublic low voltage power adapter
n
etwork that supplies buildings
u
sed for domestic purposes.
C
onducted Emissions EN 55011
C
omplies to Group 1,
C
lass B
H
armonics Emission EN 61000-3-2
C
omplies
V
oltage fluctuations / flicker
e
missions EN 61000-3-3
C
omplies
ARTEK Pro Technical Specifications
ARTEK Pro Part
Number 0V9PTATP01UNVWRC01
Operating
Environment 41F - 80F [5C - 27C]
Relative Humidity 30% to 60%, Non-
Condensing
Operating Altitude < 2000 meters
Thermal Therapy
Temperature
Range
41F - 50F [5C - 10C]
100F - 105F [37.7C – 40.5C]
Dimensions 9”H x 8”D x 9.5”W
[229mmH x 203mmD x
241mmH]
Weight 8.5 lbs [1.81 kg]
Circulatory Pump 12 VDC
Reservoir Fluid
Capacity 1.67 fl oz [50 mL]
Temperature
Accuracy +/- 3.6F [+/- 2C]
Input Voltage 48 VDC [Only use the
power adaptor provided]
Input Current
(Max) 4.5A
Recommended
Coolant 100% distilled water
ARTEK Pro Safety Signed Marked on the
Equipment

Technical Information
13
ARTEK Pro Guidance and Manufacture Declaration – Electromagnetic
Immunity
Immunity
Test
IEC 60601 Test
Level IEC 60601 Test
Level Electromagnetic Environment
- Guidance
Electrostatic
Discharge
EN 61000-4-2
+/- 8 kV contact
+/- 15 kV air
+/- 8 kV contact
+/- 15 kV air
Floors should be wood,
concrete, or ceramic tile. If
floors are covered in synthetic
material, the relative humidity
should be at least 30%.
Electrical Fast
Transient / Burst
EN 61000-4-4
+/- 2 kV for
power supply
lines
+/- 1 kV for input
/ output lines
+/- 2 kV for
power supply
lines
+/- 1 kV for input
/ output lines
Mains power quality should be
that of a typical commercial
or hospital environment.
Surge
EN 6100-4-5
+/- 1 kV line to
line
+/- 2 kV line to
earth
+/- 1 kV line to
line
+/- 2 kV line to
earth
Mains power quality should be
that of a typical commercial
or hospital environment.
Voltage dips,
short
interruptions
and voltage
variations on
power adapter
lines
EN 61000-4-11
(per EN 60601-1-
2, 4th ed)
˂5% UT, (95% dip
in UT) for 0.5
cycle
40% UT, (60% dip
in UT) for 5 cycles
70% UT, (30% dip
in UT) for 25
cycles
˂5% UT, (95% dip
in UT) for 5 sec
˂5% UT, (95% dip
in UT) for 0.5
cycle
40% UT, (60% dip
in UT) for 5 cycles
70% UT, (30% dip
in UT) for 25
cycles
˂5% UT, (95% dip
in UT) for 5 sec
Mains power quality should be
that of a typical commercial
or hospital environment.
Power frequency
(50/60 Hz)
Magnetic field
EN 61000-4-8
30 A/m (60 Hz) 30 A/m (60 Hz) Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
Conducted RF
EN 61000-4-6
Radiated RF EN
61000-4-3
3Vrms 0.15 MHz to
80 MHz
3V/m 80 MHz –
27 GHz
3Vrms 0.15 MHz to
80 MHz
3V/m 80 MHz –
27 GHz
Portable and mobile RF
communications equipment
should be used no closer to
any part of the equipment,
including cables, than the
recommended separation
distance calculated from the
equation applicable to the
frequency of the transmitter.

Keep Dry
Prescription Device
DO NOT use near explosives or an
open flame. Do not smoke around
device.
NOT
STERILE
Non-sterile
Not for general waste.
Symbols
Risk of cold injury
Refer to the instruction/user manual
Product compliance with Norh
American safety standards.
Protected against solid objects over
12.5mm (e.g., a finger) and protected
against vertically falling drops of water
or condensation
Keep away from sunlight.
Type BF applied part.
Class II
Caution
Direct current
Polarity of d.c. power connector.
Do not reuse
Catalogue number
Batch Code
Serial Number
Manufacturer
Temperature limit / Humidity limit
1.7 EMC Notice
This equipment generates, uses, and
can radiate radio frequency energy. If
not installed and used in accordance
with the instructions in this manual,
electromagnetic interference may result.
The equipment has been designed to
provide reasonable protection against
electromagnetic interference when
operated in the intended use
environments described in this manual.
1.8 Electromagnetic Interference
This device has been tested and found
to comply with the limits for Medical
Devices according to IEC60601-1-2.
These limits are designed to provide
reasonable protection against harmful
interference in typical medical
installations. This equipment
generates and radiates radio frequency
energy and, if not installed and used in
accordance with the instructions, may
cause harmful interference to other
devices in the vicinity. There is no
guarantee that interference will not
occur in a particular installation. If this
equipment does cause harmful
interference to other devices, which
can be determined by turning the
equipment on and off, the user can try to
correct the interference by one or more
of the following measures:
• Re-orient or relocate the receiving
device.
• Increase the physical separation
between the equipment and other
device(s).
• Connect the equipment into an outlet,
or circuit, different from the one where
the other device(s) are connected.
1.10 Calibration
This equipment is comprised of
components that are of high accuracy
and low drift. Under normal operation,
during its useful life, the therapy unit
does not require calibration.
1.9 MRI Notice
This equipment contains electronic and
ferrous components, whose operation
can be affected by intense
electromagnetic fields. Do not operate
the system in an MRI environment or in
the vicinity of high-frequency surgical
diathermy equipment, defibrillators, or
shortwave therapy equipment.
Electromagnetic interference could
disrupt the operation of the device.
Technical Information
14

15
The information and content within this User Manual is prprietary
and confidential to ThermoTek. It may not be reproduced or
distributed, in whole or in part, without the prior written permission
of ThermoTek.
Device Set Up and Use: 0P1PATP01QSU
Wrap Application: 0P1PATUNVWQAP
QR Code Ref:


15
Table of contents
Other ThermoTek Medical Equipment manuals