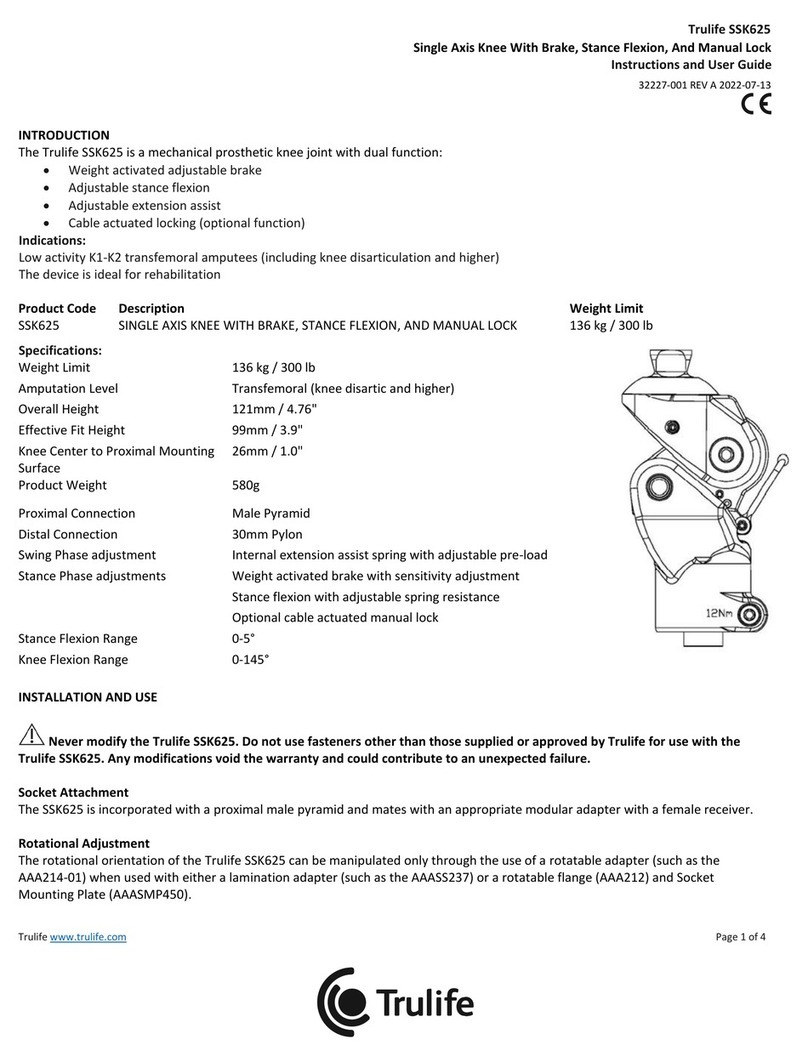
SLF190, 191,193, 195, 196,198
Seattle LightFoot² with Titanium Pyramid
Installation and User Guide
28902-001 REV B
Page 2 of 2
Trulife • 26296 Twelve Trees Lane NW, Poulsbo, WA 98370 • tel 888.878.1238 • fax 888.878.1237 • www.trulife.com
Bolt Hole Alignment
To establish anterior/posterior placement of the foot, place the ankle bolt hole 13-25 mm (1/2”-
1”) posterior to the midline of the socket. To establish medial/lateral placement of the foot,
position the ankle bolt hole in line with the midline of the proximal socket.
Above Knee Alignment
Use standard foot alignment procedures when installing the Seattle LightFoot² with Titanium
Pyramid,
MAINTENANCE GUIDELINES
• Foot assembly should be inspected after rst 30 days of use.
• Inspect entire prosthesis for wear during normal consultations.
• Periodically check the bolt for loosening. Retorque to 27Nm (20 ft-lbs or 240 in-lbs) if loose.
Warning: Looseness of foot bolt may lead to bolt failure.
QUESTIONS
Contact Customer Service in the U.S. at 888-878-1238, or fax 888-878-1237
If calling from outside the U.S., contact Customer Service at 360-697-5656,
or fax 360-697-6843. Visit Trulife online at www.trulife.com
LIMITED WARRANTY
Trulife warrants that the Seattle LightFoot² with Titanium Pyramid will be free from defects in
material and workmanship for two (2) years from the date of installation.
This warranty will not apply if the product has been damaged by misuse, abuse, neglect, improper care, failure to follow
instructions, abnormal wear and tear, or in the event that the Seattle LightFoot² with Titanium Pyramid has been modied/
repaired by persons unauthorized by Trulife.
If a defect in material or workmanship is found during the warranty period, Trulife will, at Trulife’s option, either repair or replace
the product. If it is not possible to repair or replace the product, Trulife will be limited to refunding the purchase price.
Trulife will not be liable under any legal theory for any direct, indirect, special, incidental or consequential damages arising from
the use of or inability to use this product.
The application guidelines for this Trulife product are for the use of and by certied, qualied practitioner only. Patients are not
to attempt to apply or adjust the item unless expressly instructed to do so by the practitioner responsible for the prescription
and/or initial tting of the device. All patient questions should be referred to the practitioner and not to the manufacturer. The
manufacturer warrants only that the enclosed product has been inspected for quality and can be effective for certain indications,
but nal decisions and ongoing monitoring must be made by the prosthetic professional(s) prescribing and/or tting the device
to determine its effectiveness for an individual patient. Patient compliance is an integral part of the entire protocol and must be
adhered to in order to avoid potential problems and to maximize the effectiveness of the prescribed product.
As a condition of the sale of any Trulife product, this prosthesis is restricted to a “Single Patient Use Only” by the originally tted
patient in order to protect the care provider and the patient against potentially adverse consequences of infectious disease
transmission, material instability in adapting to the conguration of the original user and/or decrease in effectivity. Any express
or implied warranties are voided if the product is reused or tted to another patient. Additionally, a license of right to use under
any relevant patents pertaining to the product is terminated with the cessation of use by the original patient. As with all Trulife
products, this product must be prescribed and applied by a qualied practitioner to determine it meets the needs of the particular
patient and accomplishes the desired results.
FIGURE 1. Alignment.
12-14 mm
1⁄2”
1/2 1/2