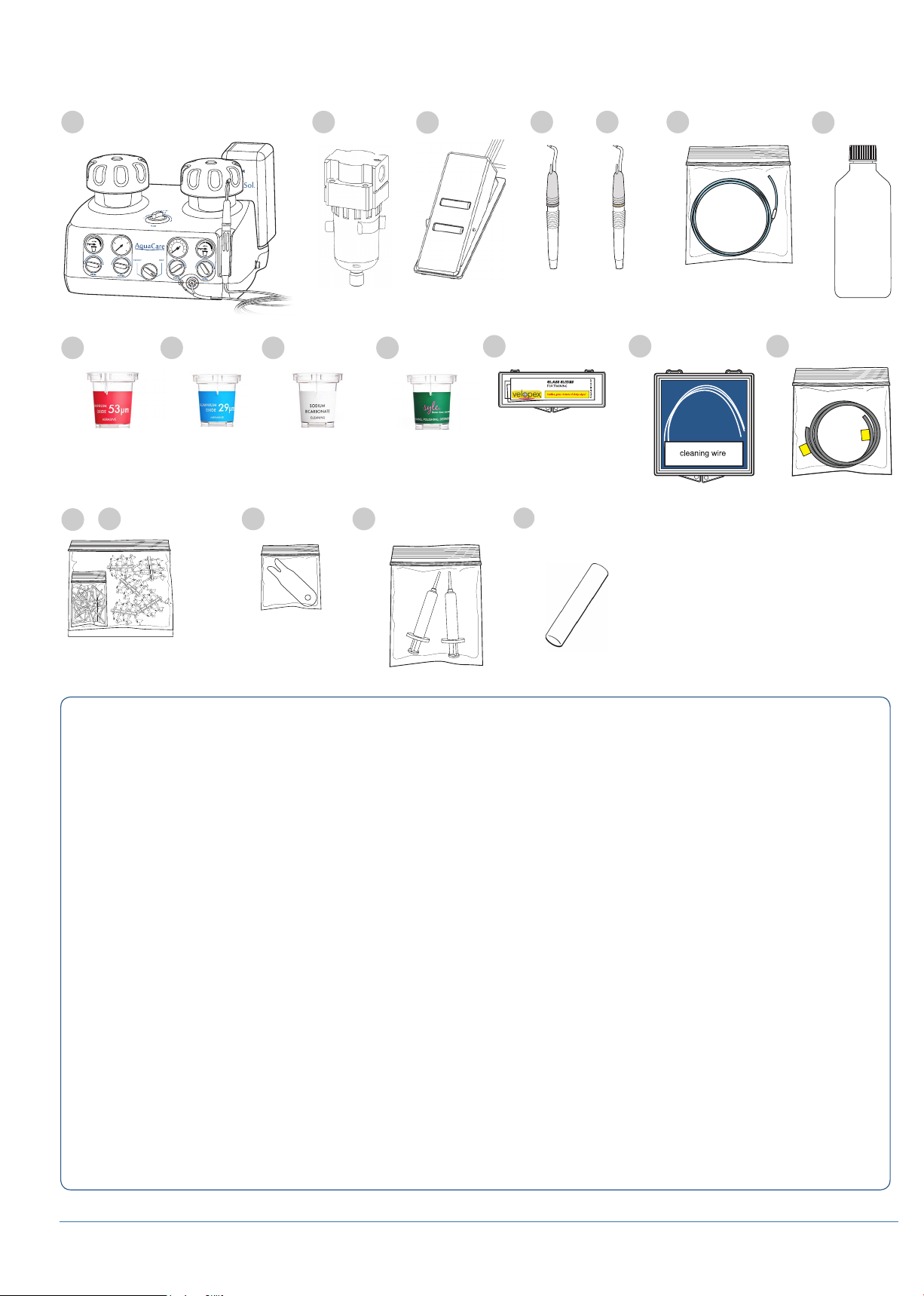
6
Safety Warnings
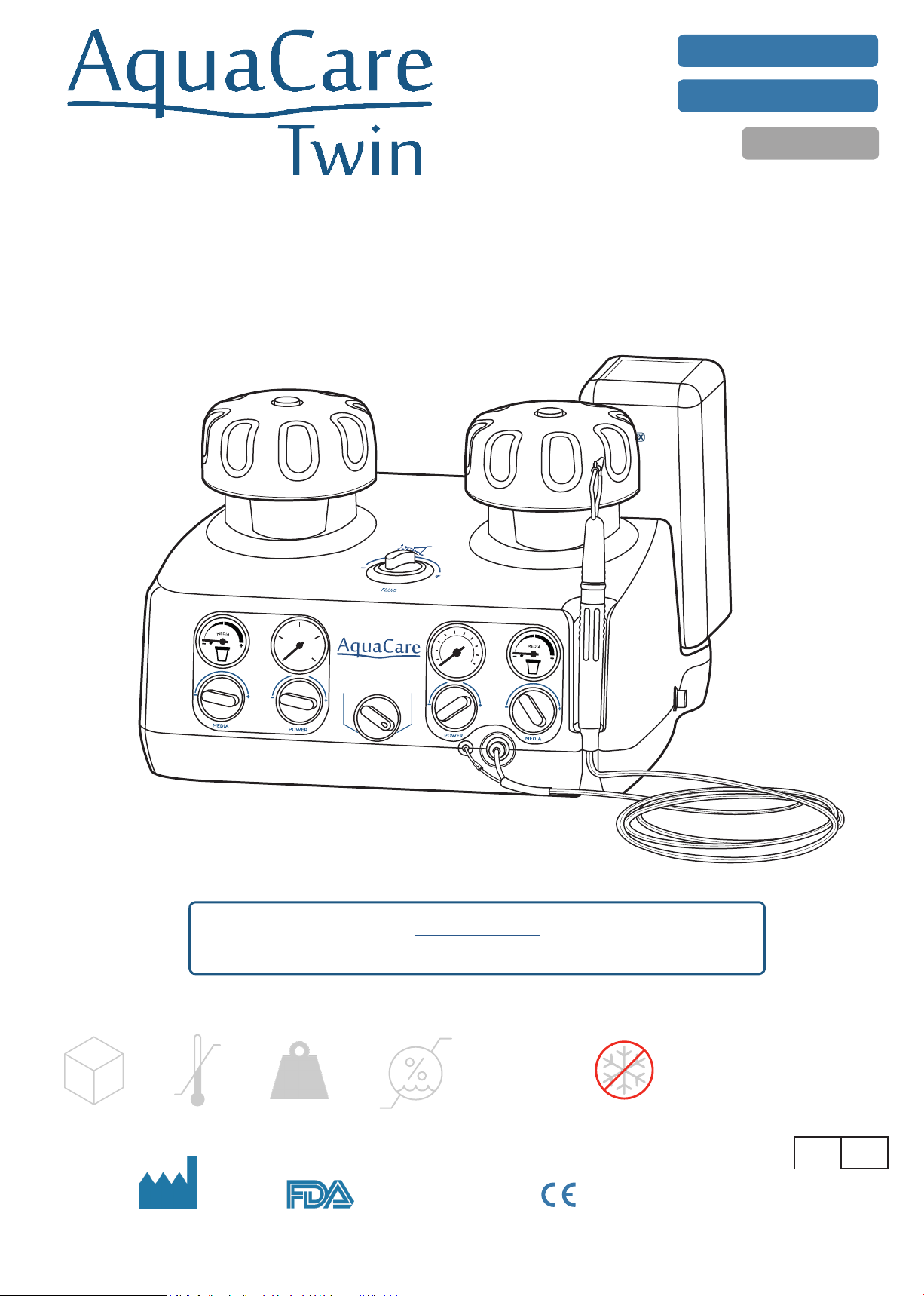
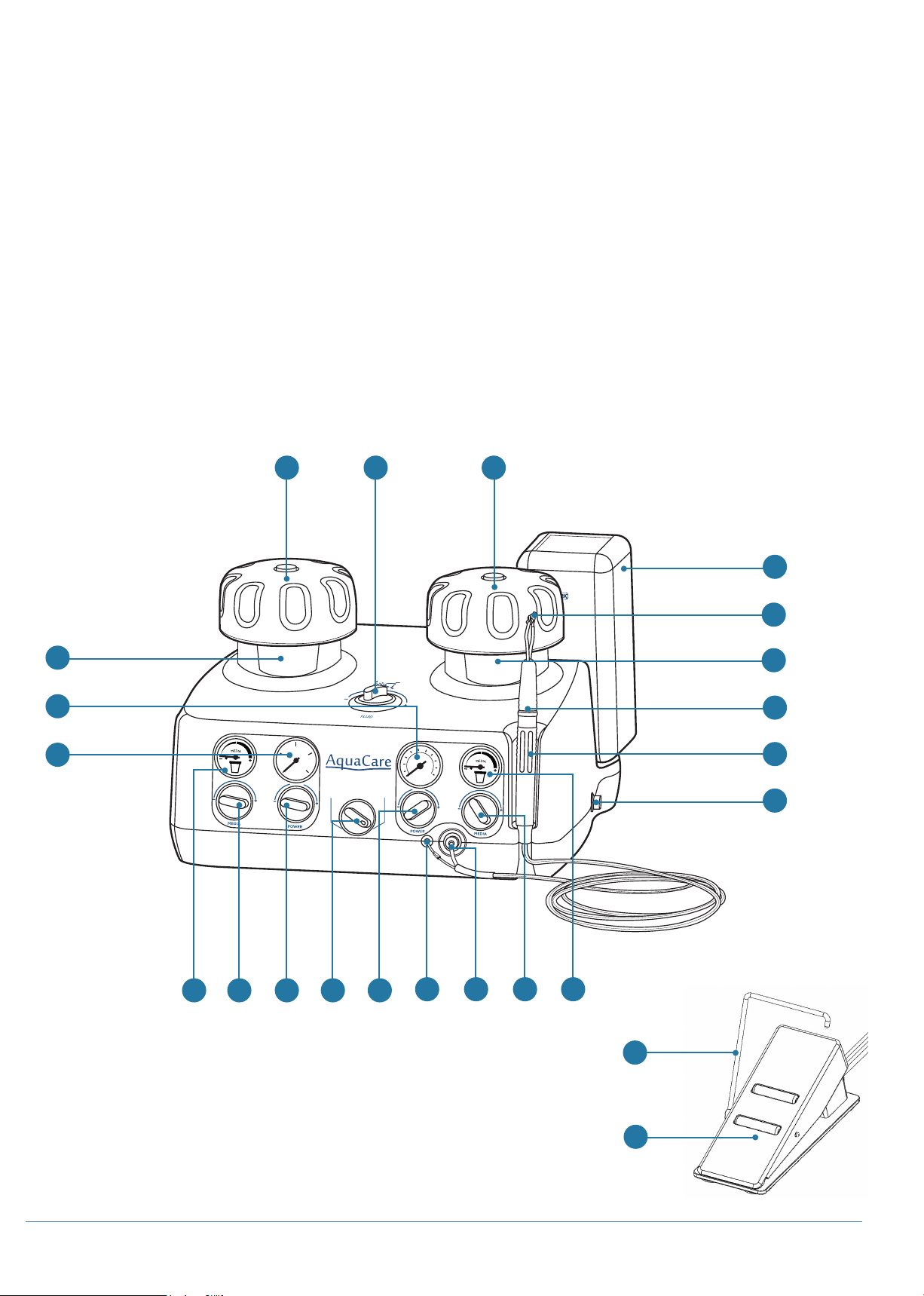
AquaCare Twin: Introduction
In most cases the plastic tip should last long enough for one treatment. However, during
prolonged treatments the tip may need replacing to prevent erratic ow of uid.Do not use
the handpiece if the hole in the tip of the nozzle has worn to the outer edge, or if the swan
neck tube has been perforated.
Sterilise the handpiece before rst use and between each patient treatment.
Fluidissuppliedtotheunitviatheuidbottle.The AquaCare Twin air inlet should
NEVER be connected to a running water supplyasthiswilloodthemachine.To
rectify this the machine will require a full service and rebuild - this will be carried out
at the customer’s expense.
All powders supplied by Velopex are sterile until opened, and the containers are not reusable.
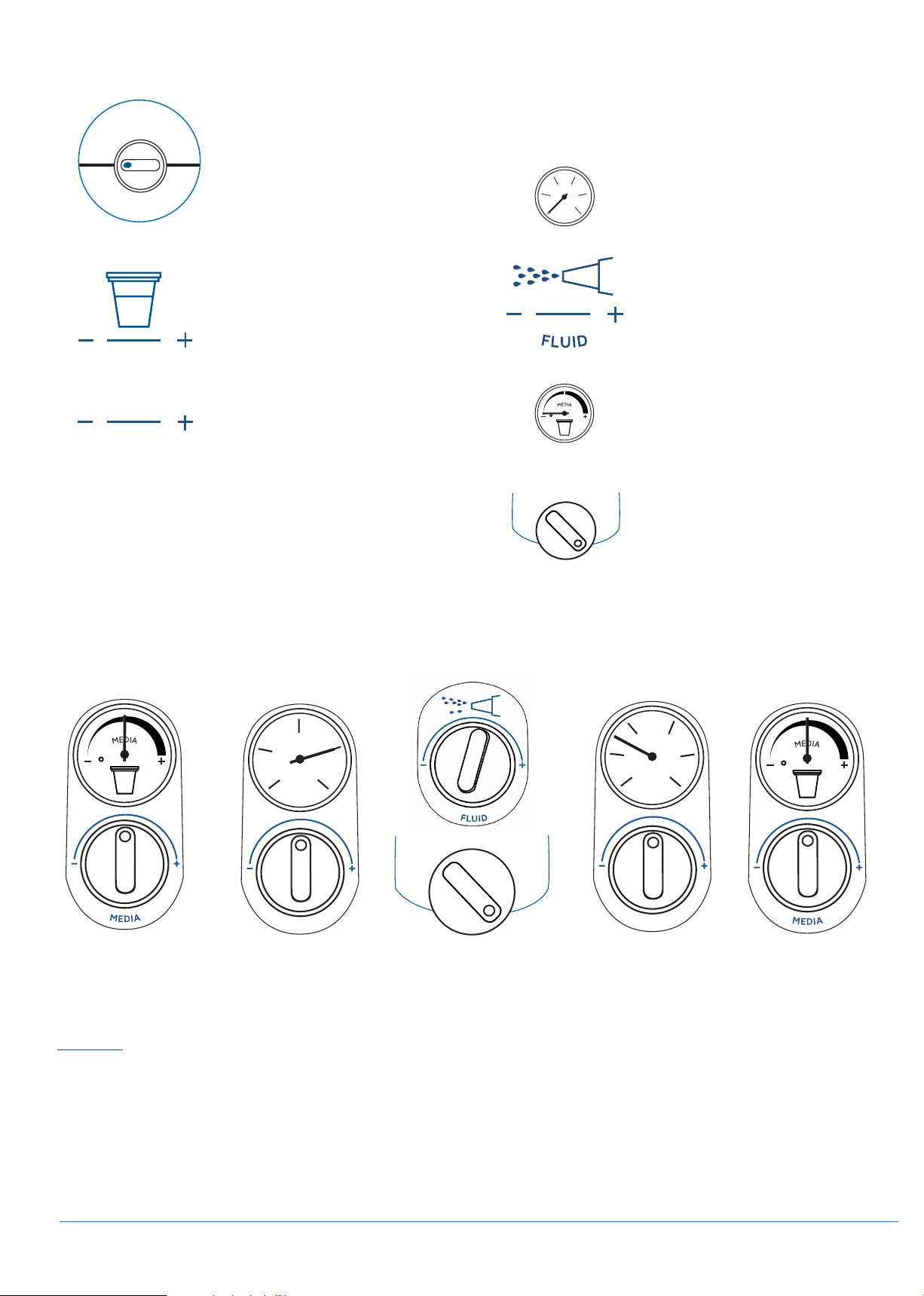
The powder lines and handpiece should be purged/primed each time you switch between
the powder chambers using the powder selector dial (See MAINTENANCE: PURGING THE
AQUACARE TWIN, Pg 33).
The AquaCare Twin produces ne powder dust when in use. Protect sensitive equipment
from this dust.
Only use parts and consumables supplied by Velopex. Non-Velopex products may damage
the unit or alter the performance. The uid used with the machine must be a biostatic
solution like the AquaSol from Velopex, see page 16.
Site the foot pedal carefully so that it cannot be operated by accident. Always close the foot
pedal safety cover when not in use. Accidental operation can cause an uncontrolled and
dangerous high pressure stream of abrasive powder.
If the foot pedal is released but the powder/uid ow does not stop therefore indicating that
the pedal has jammed in the ON position.
The rst course of action must be to quickly and carefully remove handpiece from the
patients mouth and placing the hand piece to discharge in an extraction unit.The system can
then be disabled by operating the side on/off switch to the off position.
Do not place the unit in direct sunlight.
When turning the AquaCare Twin on and off, ensure that the nozzle is aimed into the inlet of
your evacuation unit, and that the evacuation unit is switched on.
When turning the AquaCare Twin off, wait for the machine to depressurise before attempting
to remove the dosing chamber lids. Depressurising takes roughly 30 seconds.
If the AquaCare Twin is dropped or otherwise damaged, it must not be used until it has been
inspected by a Velopex Representative or Authorised Distributor.
If the AquaCare Twin ceases to operate correctly, or if you experience a
deterioration in performance, see TROUBLESHOOTING, Pg 35-37.