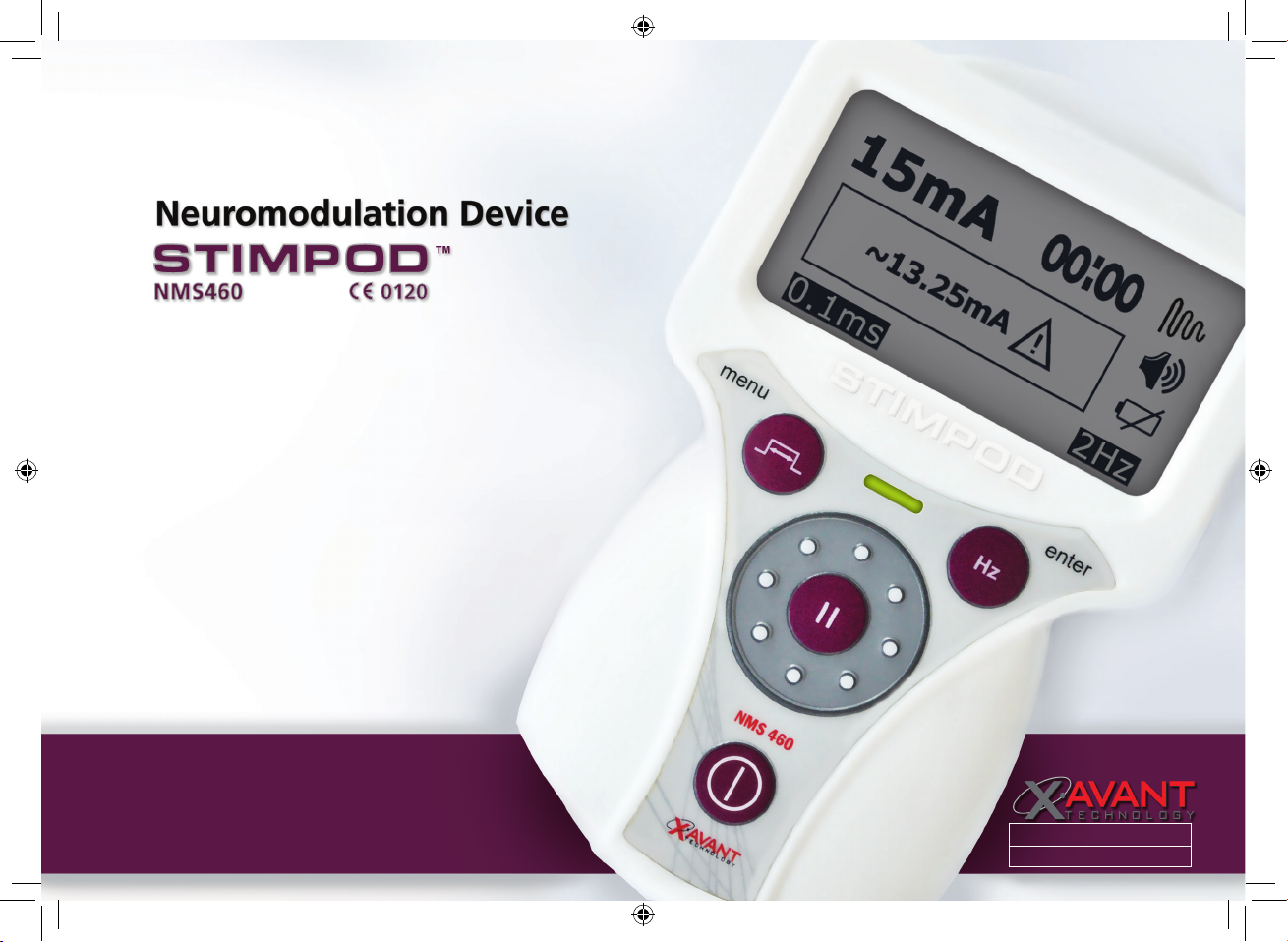
• Stimulation should not be applied across or through the head, directly on the
eyes, covering the mouth, on the front of the neck, (especially the carotid sinus),
or from electrodes placed on the chest and the upper back or crossing over the
heart.
• Use of accessories, transducers and cables other than those specified or provided
by the manufacturer of this equipment could result in increased electromagnetic
emissions or decreased electromagnetic immunity of this equipment and result in
improper operation.
• Do not apply stimulation over the menstruating or pregnant uterus.
Cautions:
• Remove elements which may adversely affect the connection between the ECG
electrodes and the skin, e.g., dirt, hair, oil.
• Ensure that ECG electrodes are not damaged or dried out.
• ECG electrodes may be used in the higher current ranges (> 10 mA).
• This product must be stored at room temperature.
• This product must be transported in the carry case provided.
• This product and all the accessories have been certified latex free.
• Inspect all parts for any damage or manipulation. Never use any damaged or
manipulated part!
• If an electrically conductive surface of the STIMPOD NMS460 device or its cables
are exposed, such electrically conductive surface may shock a person handling it.
Do not use such a device or accessory, please contact the manufacturer for repair.
• A sterile wipe should be used to disinfect the Nerve Mapping Probe prior to use.
• Keep this device out of reach of children.
• Use this device under the continued supervision of a licensed practitioner.
Precautions:
• Electrical nerve stimulators are not effective for pain of central origin, including
headache caused by central origin.
• Electrical nerve stimulators are not a substitute for pain medications and other
pain management therapies.
• Electrical nerve stimulator devices have no curative value.
• Electrical nerve stimulators are a symptomatic treatment and, as such, suppresses
the sensation of pain that would otherwise serve as a protective mechanism.
• Effectiveness is highly dependent upon patient selection by a practitioner qualified
in the management of pain patients.
• The long-term effects of electrical stimulation are unknown.
• Since the effects of stimulation of the brain are unknown, stimulation should not
be applied across the head, and electrodes should not be placed on opposite sides
of the head.
• The safety of electrical stimulation during pregnancy has not been established.
• Some patients may experience skin irritation or hypersensitivity due to the
electrical stimulation or electrical conductive medium (gel).
• Patients with suspected or diagnosed epilepsy should follow precautions
recommended by their physicians.
• Use caution when patient has a tendency to bleed internally, such as following
an injury or fracture.
• Use caution following recent surgical procedures when stimulation may disrupt
the patient’s healing process.
• Use caution if stimulation is applied over areas of the skin that lack normal
sensation.
Adverse Reactions:
• Patients may experience skin irritation and burns beneath the stimulation
electrodes applied to the skin.
• Patients may experience headache and other painful sensation during or following
the application of electrical stimulation near the eyes and to the head and face.
• Patients should stop using the device and should consult with their physicians if
they experience adverse reactions from the device.
Warranty:
• The STIMPOD NMS460 carries a 12 Month Warranty against defects, provided
that the device was used in accordance with the operating instructions.
• The STIMPOD NMS460 enclosure should not be opened under any circumstances.
Opening the unit will void the warranty.
Note:
• The STIMPOD NMS460 is not intended for home use.
STIMPOD 460 conforms to the following standards:
• IEC 60601-1, IEC 60601-2-10
• IEC 60601-1-2: CISPR 11 Group1 class B; IEC 61000-4-2; IEC 61000-4-3
• ISO 13485, Directive 93-42-EEC, ISO 9001
iii