ADInstruments Human NIBP Nano User manual

Human NIBP
Nano
Quick Start
Get set up and start recording
Non-invasive hemodynamics

6479-D

This guide will help you to quickly set up the Human NIBP Nano System. For detailed
installation and set up instructions and troubleshooting information, please refer to
the Owner’sGuide (also available at www.adinstruments.com/support/manuals).
The Human NIBP Nano System contains the following:
• Human NIBP Nano Wrist Unit
• Height Correction Unit
• LabChart Pro® and Human NIBP Device Enabler soware CDs
• Human NIBP Nano Interface and USB cable and power cable
The Human NIBP Nano Wrist Unit is manufactured by Finapres Medical Systems
(FMS) B.V. (www.finapres.com) for distribution by ADInstruments, and for use with
ADInstruments soware. Finger cus are purchased separately:
• Finger Cu (Large, for finger circumferences 65-75 mm)........................... MLT382/L
• Finger Cu (Medium, for finger circumferences 55-65 mm)....................... MLT382/M
• Finger Cu (Small, for finger circumferences 45-55 mm)........................... MLT382/S
System Requirements
Windows
• A PC capable of running LabChart 8.1.8 or above. For detailed system
requirements please see https://www.adinstruments.com/support/system-
requirements.
PowerLab not required
• An ADInstruments PowerLab is not required to use the Human NIBP Nano System
with LabChart, but you can record data simultaneously into LabChart using a
PowerLab and the Human NIBP Controller.
Overview

The Human NIBP System consists of the following hardware components:
The Human NIBP Nano Wrist Unit
• The Wrist unit is the core of the system. It has input connectors for up to 2
finger cus as well as a the HCU Unit. The long output cable connects the
Human NIBP Nano Interface.
Height Correction Unit (HCU)
• This corrects for hydrostatic pressure changes when the measured hand moves
away from heart level. The HCU consists of a liquid-filled tube connected at
one end to a pressure transducer.
Human NIBP Nano Interface
• The Human NIBP Nano Interface provides power and USB connectivity to the
Wrist Unit. It has a Redel input at the front for connection to the wrist unit and
an IEC power input and USB type B input at the rear. It is supplied with a USB
cable and a power cable appropriate for your country.
What’s in the box
HCU transducer – attach at finger level Reference end – attach at heart level
Connection to Wrist unit
en
en
n
d
d
Power Indicator Redel Connector input
Human NIBP
Nano Interface
Input
Status
r
r
r
ta
tat
at
at
t
us
us
u
u
u
t

Connect Wrist Unit to Interface
1.Ensure the Redel cable is connected securely to the front panel of the Human
NIBP Nano Interface.
Connect device to your computer
2.Connect the Human NIBP Nano Interface to your
computer using the supplied USB cable.
3.Connect the power cord to a grounded power supply and
to the rear panel of the Human NIBP Nano Interface.
Power on the hardware
Turn on the Human NIBP Nano Interface using the power switch on the rear panel.
1. Unpack the box
Power switch
Connect USB to computer Connect to mains power
USB cable
Power cable
I
O
Made in Australia by ADInstruments Pty Ltd.
100 - 240 VAC, 50/60 Hz, 20 VA
Power
ow
w
w
w
w
w
er
e
e
e
e
0
0
Hz
Hz
H
H
H
H
H
H
z
z
z
z
z,
ra
a
a
a
li
li
i
a
a
a
a
a b

GLP Client
LabChart®Installers
The Human NIBP Nano System consists of the following soware components:
LabChart Pro®
Human NIBP Device Enabler CD
1.The Human NIBP Device Enabler is a soware addition to LabChart. It is designed
to allow LabChart to recognise the Human NIBP Controller, when it is connected
to your computer, and to open LabChart with the appropriate settings to make
non-invasive blood pressure measurements.
2.Place the Human NIBP Device Enabler CD in the CD drive of your computer.
Alternatively you can download the latest version of the Human NIBP Device
Enabler from here (https://www.adinstruments.com/support/soware)
3.The installer should auto-run. If it doesn’t, see the Read Me on the CD.
4.Alternatively, use LabChart’s Feature Manager
(Help> FeatureManager...) to download the latest
installer.
2. Install soware
6
1
1
4-
A
Human NIBP Device Enabler
1.Place the LabChart Pro CD in the CD drive of
your computer. Alternatively you can download
the latest version of LabChart from here (https://
www.adinstruments.com/support/downloads/
windows/labchart)
2.Follow the on-screen instructions to install
LabChart 8.
3.Launch LabChart by double-clicking on the
desktop icon. Enter the activation code when prompted.
4.Check for updates (Help> Check for Updates...) and install the latest LabChart

License Registration
1.When you start LabChart for the first time, a dialog will appear asking you to
enter your license code. Your license code can be found on the LabChart CD
case. If you purchased LabChart Pro then both codes on the back of your CD
case should be entered in turn to unlock all the features of LabChart Pro.
2.Enter your license details.
Device Discovery
On start-up, LabChart performs a Device Discovery process. It should automatically
detect the Human NIBP Nano device which will be indicated by a green tick mark. It
is recommended that the Human NIBP Nano
Interface is powered on BEFORE connecting the
Human NIBP Wrist Unit to the Interface. If the
device is not detected immediately click Device
Scan. If detection continues to fail aer repeated
device scans see the Troubleshooting section in
the Owner’s Guide.
License Activation
Activation is only required once per machine. If a supported PowerLab is already
connected and turned on, your license will activate automatically. Otherwise, you
may choose online or manual activation.
Welcome to LabChart!
LabChart’s Welcome Center opens automatically when LabChart is launched.
1.In the Getting Started tab of the Welcome Center, double-click to open the
Human NIBP settings file.
2.You should see the device name appear at the top of the new document.
3.The Start button should be enabled and you are now ready to start recording.
3. Start LabChart
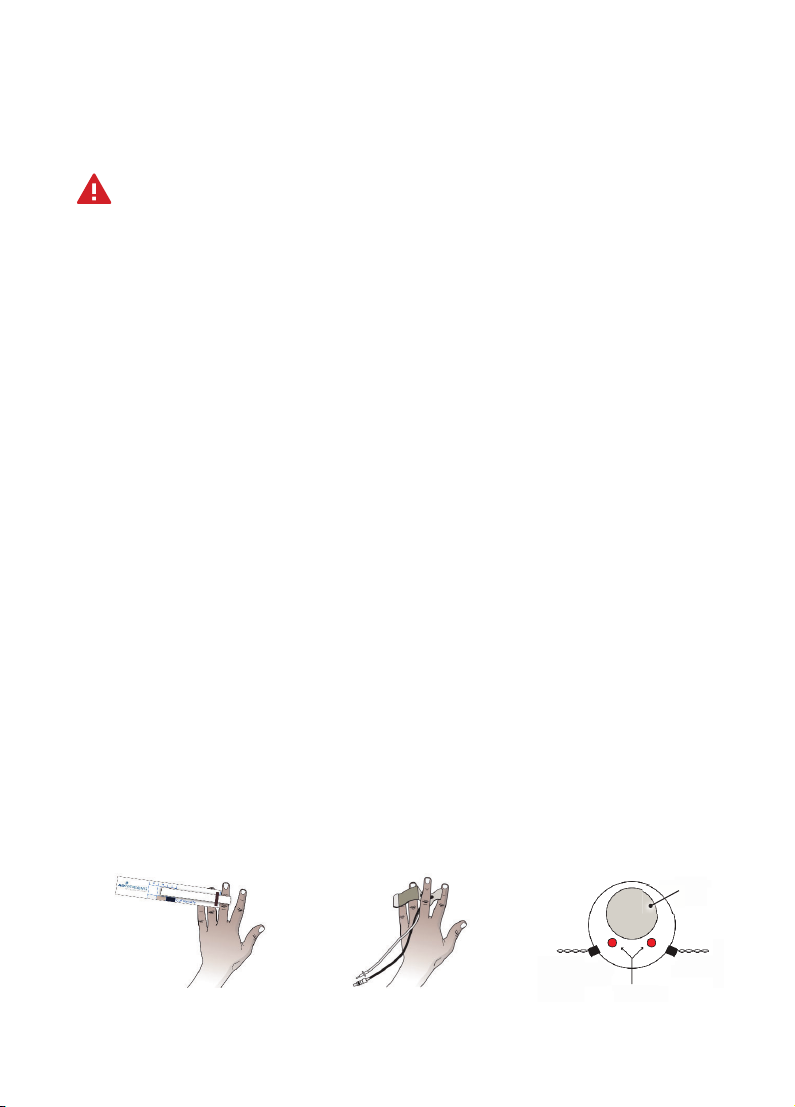
Apply finger cuff(s)
Warning!Finger cus will be damaged if allowed to inflate without a finger (or
another, appropriately sized object) inside them.
Proper finger cu application is critical for success with the Human NIBP Nano. Select
the proper finger cu size. If in doubt, choose the smaller finger size cu.
1.Open the finger cu just enough to be able to see the LED and photodiode (or
photocell, PC).
2.Place the middle, index or ring finger in the cu. The LED and photocell should
be symmetrically placed on each side of the finger’s so parts in the center of the
middle phalanx.
3.Lead the cu cable and air hose in between two fingers to the back of the hand.
Here these connect to the Wrist Unit.
4.Make sure the finger cu is placed centered between two knuckles, touching each
knuckle.
5.Wrap the finger cu tightly for best performance. A common mistake is to not
wrap the finger cu tightly enough.
6.If finger cu switching is to be used, repeat steps 1–5 to apply a finger cu to a
second finger. Finger-switching is recommended for measurements of 1 hour or
longer.
4. Before starting a measurement
1. Select the correct
cu using the cu
size guide.
2. Wrap the cu tightly,
preferably around the
middle finger, between the
joints. Make sure the cable
and tube point towards the
wrist.
3. Center the LED
and photocell
symmetrically
around the finger.
Finger cross-section
Arteries
LED light emitting diode PC photocell
Bone
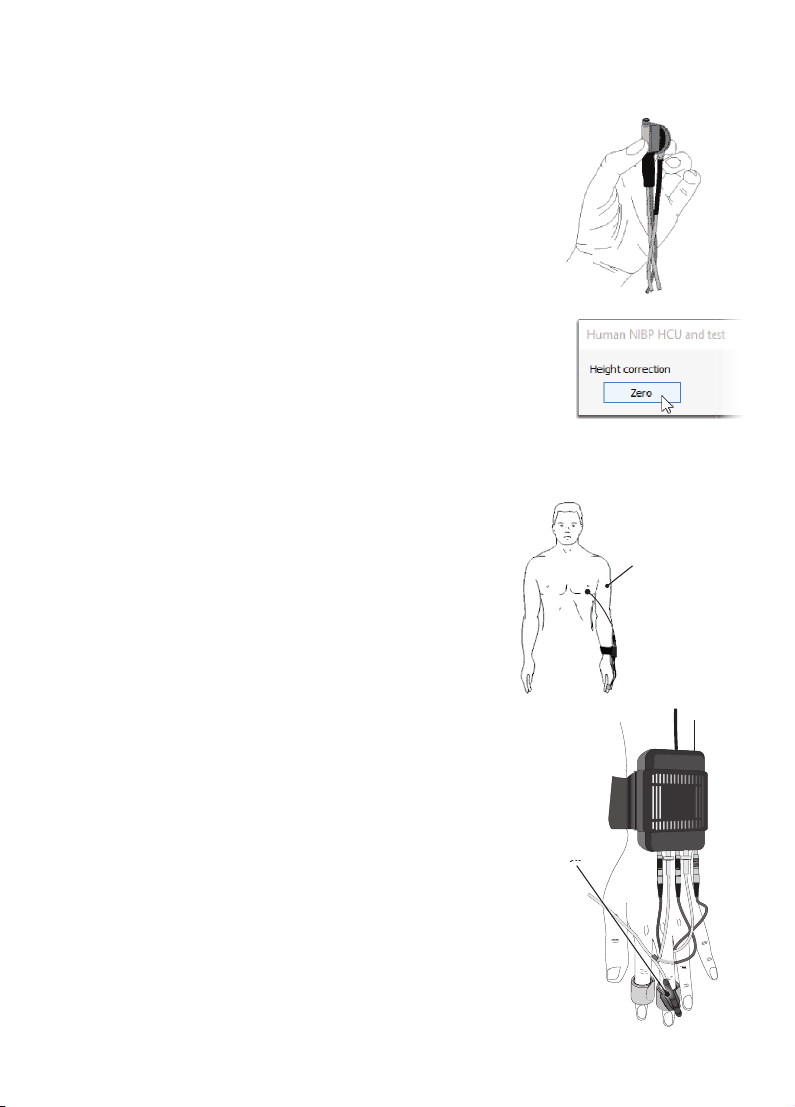
Instructions
Zero the HCU
Perform the height correction procedure before attaching
the HCU Unit to the volunteer.
1.Hold the HCU transducer and the reference point at
the same level.
2.In LabChart, click Setup > NIBP HCU andtest...
3.In the Human NIBP Settings dialog, click Zero.
Once the HCU has successfully been zeroed a confirmation
message is displayed.
The Wrist Unit is connected to the Human NIBP Controller
by a cable assembly.
1.Apply the Wrist Unit to the volunteer’s wrist and
fasten the strap firmly so that it cannot twist.
2.Guide the cable assembly along the arm and
fasten the arm straps.
3.Insert the cu cable and air hose connectors for
each finger cu into the Wrist Unit.
4.Ensure the HCU is connected to the Wrist Unit.
5.The HCU transducer should be attached to the
finger cu and the reference end should be
attached at heart level.
Apply the Wrist Unit and HCU
C1 HCU C2
Reference end
HCU transducer
duc
duc
u
er

In LabChart, click Setup > NIBP Settings... to open the Settings dialog. The default
options are a good starting point.
1.Auto-calibration (or AutoCal) – is an ongoing calibration, which greatly
improves the accuracy measurements.
• AutoCal is performed frequently at the start of a measurement but – once the
correct, unloaded diameter of the finger artery is established – is repeated only
once every 70 heart beats.
• If the volunteer is moving around a lot, such as during exercise, the AutoCal
function does not improve accuracy and should be turned o.
2.Finger cu switching – select ‘switch cus’, and assign the first cu to start
measuring and between 4 switching periods (1, 15, 30 and 60 minutes), or choose
to measure a specific cu Cu1 (C1) or Cu2 (C2).
• For measurements longer than 1 hour always select finger switching, since a
prolonged measurement on one finger can be unpleasant for the volunteer.
• The 1-minute interval is for testing purposes only.
• Switching interval can be changed at any time.
5. Choose Human NIBP settings
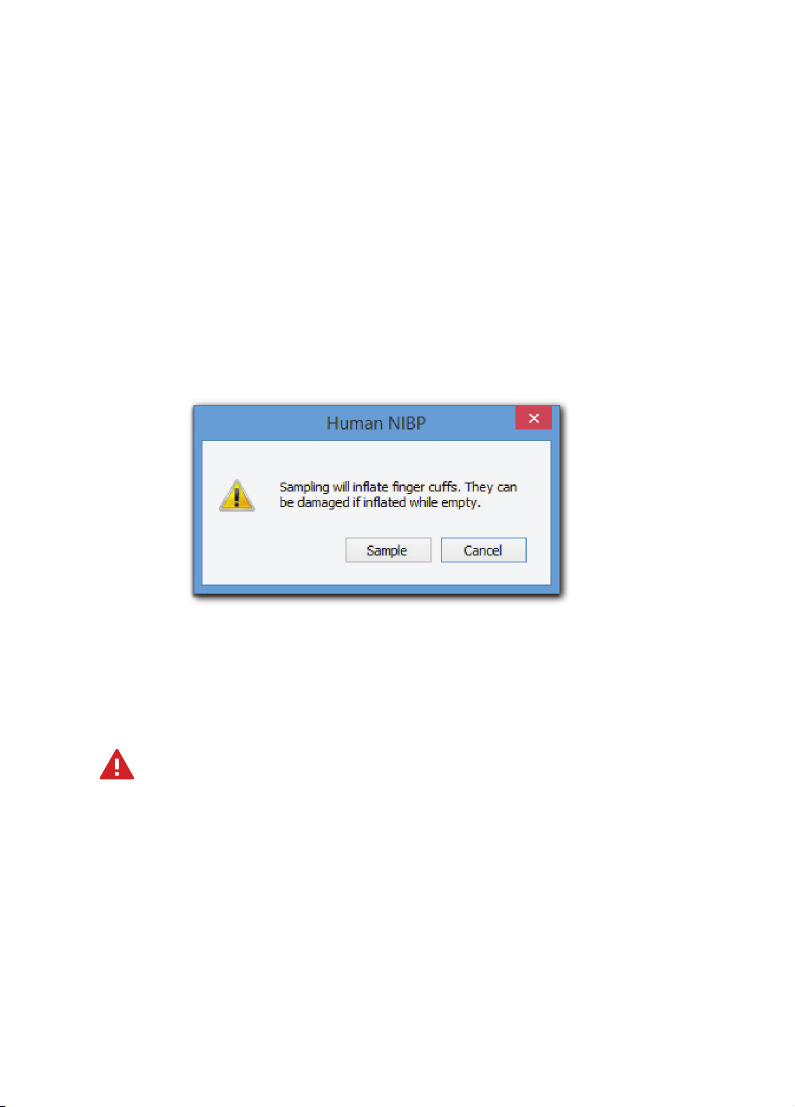
To start a blood pressure measurement simply click Startin LabChart.
Before you start a measurement...
1.Check that finger cu(s) are applied and that these are not easy to rotate.
2.Make sure the HCU has been zeroed and is attached to the volunteer.
3.Ensure you have selected your settings for the finger blood pressure
measurement in the NIBP Settings dialog.
The first time you click Start, the following Warning dialog will appear:
• To proceed, click Sample.
• To delay a measurement, click Cancel.
• This dialog will not reappear within the same LabChart session.
Warnings to prevent finger cu damage:
• Don’t apply air pressure to a finger cu when it is not wrapped around a finger
(or any other solid object!) as this will damage the finger cu.
• Don’t remove the finger cu from a finger before stopping the measurement or
before disconnecting the air hose from the Wrist Unit.
• Don’t bend finger cus outwards into a flat shape.
• Don’t attempt to repair defective finger cus as this will substantially aect
measurement accuracy.
6. Start a measurement

To stop a measurement, simply click Stop in LabChart. Once you have stopped a
measurement, it is recommended to:
1.Disconnect the finger cu air hoses from the Wrist Unit – this removes residual
air pressure from the finger cus and protects them from accidental inflation and
damage.
2.Unwrap and remove the finger cus(if you have completed your measurement).
3.Inspect the finger aer removing a finger cu – see if you can find the places
where the LED and photodiode have pressed on the finger skin (see Figure below).
• Verify that the pressure points are correctly centered between the knuckles and
symmetrically placed on opposite sides of the finger.
• The most common reason for inaccurate finger blood pressure readings is
incorrect cu application.
• If a cu is applied too tightly, an unrealistically low reading may result.
• Conversely, if the cu is applied too loosely, unrealistically high blood pressure
readings may occur.
7. Stop a measurement
Cus are used to measure blood pressure at the finger. Correct sizing and
positioning are important to obtain reliable measurement results.
Incorrect
Cable and tube
point in the wrong
direction
Incorrect
Cu is rotated
Incorrect
Cu is too close to
the hand

1.If the Human NIBP Nano is returning error messages or failing to start a
measurement, it is recommended to:
• Disconnect the finger cu air hoses from the Wrist Unit.
• Shut down LabChart.
• Turn the Human NIBP Nano Interface o and back on.
• Unplug the USB cable, then reconnect it.
• Reapply the finger cu(s).
• Restart LabChart before resuming a measurement.
2.Operating conditions
• The Human NIBP Nano has a narrow temperature operating range (10–40°C)
and should be placed apart from other equipment for optimal operation.
• It is best to perform measurements at room temperature.
• It is important to keep the hand warm during ambulatory measurements.
• If blood pressure is measured on cold fingers, the measurement can be diicult
or even impossible.
3.Height correction
• The height reference level should be selected carefully. If the subject is walking
or sitting it is best to place the reference part on the chest at the level of the
right atrium in the mid-axillary line.
• In the recumbent (lying) position it is best to place the reference ending at mid-
chest level.
• If the experiment focuses on the dynamic response of the hemodynamic system
aer a change in posture it is usually best to keep both the transducer and the
reference ending at the level of the right atrium to avoid a dynamic response of
the height correction system.
• As a general rule, try to keep the cued fingers approximately at heart level,
even with a connected height correction unit. The HCU pressure should not
exceed ±20 mmHg.
• The HCU tubing should be free of bends and unobstructed in any way.
8. Troubleshooting

Statement of intended use
All products supplied by ADInstruments are intended for use in teaching and research
applications and environments only.
ADInstruments products are NOT intended to be used as medical devices or in medical
environments. That is, no product supplied by ADInstruments is intended to be used
to diagnose, treat or monitor a subject. Furthermore no product is intended for the
prevention, curing or alleviation of disease, injury or handicap.
This product meets the IEC60601-1 standard, under the principle that:
• it is a more rigorous standard than other standards that could be chosen.
• it provides a high safety level for subjects and operating personnel.
Avoiding injury to subjects and personnel
• Finger cus shouldn’t be applied to any body part other than the fingers.
• Do not over tighten the Wrist Unit strap as that will cut o circulation to the
hand, leading to discomfort and measurement errors.
• Do not wrap finger cus around a toe or the wrist of an infant.
• For safe and reliable operation and optimal accuracy only use finger cus
manufactured by ADInstruments and FMS.
• Physiological signals acquired by other devices, such as respiratory signals and
ECGs, can be connected to the ADInstruments PowerLab system for recording
while making blood pressure measurements using the Human NIBP Controller.
• Note that any connected equipment has to meet the IEC specifications
(IEC601 for electromedical devices or IEC950 for data processing devices). The
configuration has to meet the IEC system standard (IEC601-1-1). Connection of
additional devices implies a responsibility to adhere to the IEC601-1-1 standard.
• If any of the components of the Human NIBP System appear damaged in any
way, please contact your ADInstruments® representative.
Further resources

Product Purchase and License Agreement
This Agreement is between ADInstruments NZ Ltd [‘ADI’] and the purchaser [‘the Purchaser’]
of any ADI product or solution — software, hardware or both — and covers all obligations and
liabilities on the part of ADI, the Purchaser, and other users of the product. The Purchaser (or
any user) accepts the terms of this Agreement by using the product or solution. Any changes
to this Agreement must be recorded in writing and have ADI’s and the Purchaser’s consent.
Responsibilities
The Purchaser and any others using any ADI product or solution agree to use it in a sensible
manner for purposes for which it is suited, and agree to take responsibility for their actions
and the results of their actions. If problems arise with an ADI product, ADI will make all
reasonable efforts to rectify them. This service may incur a charge, depending on the nature
of the problems, and is subject to the other conditions in this Agreement. ADI does not
separately warrant the performance of products, equipment or software manufactured by
third parties which may be provided to Purchaser as part of an overall solution. However, as
further noted below, ADI will pass through to Purchaser all applicable third party warranties
to the extent it has the right to do so.
Statementof Intended Use
Products supplied by ADInstruments are intended for use in teaching and research
applications and environments.
Products supplied by ADInstruments are NOT intended to be used as medical devices or in
medical environments. That is, no product supplied by ADInstruments is intended to be
used to diagnose, treat, or monitor a subject. Furthermore no product is intended for the
prevention, curing or alleviation of disease, injury or handicap.
Where a product meets IEC 60601-1 it is under the principle that:
1. it is a more rigorous standard than other standards that could be adopted and
2. it provides the most appropriate safety level for subjects and operators.
The choice to meet IEC 60601-1 is in no way to be interpreted to mean that a product:
1. is a medical device;
2. may be interpreted as a medical device;
3. is safe to be used as a medical device
ADI Product HardwareWarranty
ADI warrants that PowerLab Data Acquisition Units (PL prefix)1 and Front-ends (FE prefix)2
shall be free from defects in materials and workmanship for five (5) years from the date of
purchase. Other PowerLab Data Acquisition Units3, Front-ends4and Pods5shall be free of

defects in material and workmanship for three (3) years from their date of purchase. ADI
also warrants that ADI Specialized Data Recorders6and Instruments7shall be free of defects
in material and workmanship for one (1) year from their date of purchase. If there is such
a defect, as Purchaser’s sole remedy hereunder, ADI will repair or replace the equipment
as appropriate, and the duration of the warranty shall be extended by the length of time
needed for repair or replacement.
To obtain service under this warranty, the Purchaser must notify the nearest ADI office,
or Authorized Representative, of the defect before the warranty expires. The ADI or
Representative office will advise the Purchaser of the nearest service center address to
which the Purchaser must ship the defective product at his or her own expense. The product
should be packed safely, preferably in its original packaging. ADI will pay return shipping
costs.
HardwareWarranty Limitations
This warranty applies only to the ADI hardware specified in this document and used
under normal operating conditions and within specification. Consumables, electrodes
and accessories are not covered by this warranty. Third party equipment may be covered
by the third party manufacturer’s warranty. To the extent that ADI has the right to pass
through any third party manufacturer warranties to Purchaser it will do so to the extent
it is able to do so. Copies of applicable third party manufacturer warranties, to the extent
they exist, are available upon request. The warranty provided hereunder does not cover
hardware modified in any way, subjected to unusual physical, electrical or environmental
stress, used with incorrectly wired or substandard connectors or cables, or with the original
identification marks altered. Tampering with or breaking of the Warranty Seal will also void
the warranty.
Product Types & Warranty Term
ADI manufactured products covered by five (5) year warranty
1. Data Acquisition Units: PowerLab 35 series with PL prefix.
2. Front-ends: ADI Front-end Signal Conditioners with FE prefix.
ADI manufactured products covered by three (3) year warranty
3. Data Acquisition Units: PowerLab 26 series with ML prefix.
4. Front-ends: ADI Front-end Signal Conditioners with ML prefix.
5. Pods: The entire range of ADI Pod Signal Conditioners.
ADI manufactured products covered by one (1) year warranty
6. Specialized Data Recorders: Metabolic Systems (e.g. ML240 PowerLab/8M Metabolic
System)

7. Instruments: Blood FlowMeter, Gas Analyzers, NIBP System (excluding transducers),
STH Pump Controller.
Third Party Products (Including Transducers)
Products not manufactured by ADI are covered by the manufacturer’s warranty.
Accessories and Consumables
Accessories and Consumables are not covered by any type of warranty.
General Limitations
ADI products are produced to high standards, and should perform as described in the
supplied documentation. There is a limited hardware warranty, and technical support is
provided for all ADI products. Nevertheless, since ADI products could be affected by external
factors (for instance, the computer system on which they run and other hardware and/or
software provided by third parties), absolute performance and reliability of products and
the overall solution cannot be guaranteed. No warranty, either expressed or implied or
statutory, other than that expressly contained in this Agreement, is made in respect to ADI
products or software, third party products or software, the overall solution or otherwise.
The Purchaser therefore assumes all risks as to the performance and reliability of the
products, the software, the solution and the results gained using them. ADI neither assumes
or authorizes any person to assume on its behalf any liability in connection with the sale,
installation, service or use of its products. ADI shall not be held responsible for special,
consequential or punitive damages of any kind arising out of sale, installation service or use
of its products.
EXCEPT FOR THE EXPRESS WARRANTY SET FORTH HEREIN, THE SOLUTION AS WELL AS
ALL EQUIPMENT AND SOFTWARE PROVIDED HEREUNDER ARE PROVIDED “AS IS” AND ADI
MAKES NO WARRANTY AS TO ITS USE OR PERFORMANCE. EXCEPT FOR ANY WARRANTY,
CONDITION, REPRESENTATION OR TERM THE EXTENT TO WHICH CANNOT BE EXCLUDED OR
LIMITED BY APPLICABLE LAW, ADI AND ITS SUPPLIERS MAKE NO WARRANTY, CONDITION,
REPRESENTATION, OR TERM (EXPRESS OR IMPLIED, WHETHER BY STATUTE, COMMON LAW,
CUSTOM, USAGE OR OTHERWISE) AS TO ANY MATTER INCLUDING, WITHOUT LIMITATION,
NON INFRINGEMENT OF THIRD PARTY RIGHTS, MERCHANTABILITY, INTEGRATION, OR
FITNESS FOR A PARTICULAR PURPOSE. YOU ASSUME RESPONSIBILITY FOR SELECTING THE
SOLUTION TO ACHIEVE YOUR INTENDED RESULTS, AND FOR THE INSTALLATION OF, USE OF,
AND RESULTS OBTAINED FROM THE EQUIPMENT AND SOFTWARE. WITHOUT LIMITING THE
FOREGOING PROVISIONS, ADI MAKES NO WARRANTY THAT THE EQUIPMENT OR SOFTWARE
WILL BE ERROR-FREE OR FREE FROM INTERRUPTIONS OR OTHER FAILURES OR THAT THE
SOFTWARE OR EQUIPMENT WILL MEET YOUR REQUIREMENTS.

UNDER NO CIRCUMSTANCES AND UNDER NO LEGAL THEORY, WHETHER IN TORT, CONTRACT,
OR OTHERWISE, SHALL ADI OR ITS SUPPLIERS BE LIABLE TO PURCHASER OR TO ANY
OTHER PERSON FOR LOSS OF PROFITS, LOSS OF GOODWILL, OR ANY INDIRECT, SPECIAL,
INCIDENTAL, OR CONSEQUENTIAL DAMAGES, OR DAMAGES FOR GROSS NEGLIGENCE OF
ANY CHARACTER INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF GOODWILL,
WORK STOPPAGE, COMPUTER FAILURE OR MALFUNCTION, OR FOR ANY OTHER DAMAGE OR
LOSS. IN NO EVENT SHALL ADI OR ITS SUPPLIERS BE LIABLE FOR ANY DAMAGES IN EXCESS
OF THE PRICE PAID FOR THE EQUIPMENT AND SOFTWARE, EVEN IF ADI, OR ITS AUTHORIZED
PARTNERS OR SUPPLIERS HAVE BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
ADI is acting on behalf of its suppliers for the purpose of disclaiming, excluding and/or
limiting obligations, warranties and liability as provided in this agreement, but in no other
respects and for no other purpose. The foregoing provisions shall be enforceable to the
maximum extent permitted by applicable law.
Controlling Law and Severability
This license shall be governed by the laws of the territory into which the software is sold, or
if sold into the United States of America, by the laws of the State of California.
Technical Support
The Purchaser is entitled to free technical support for any ADI product for one year from its
date of purchase. Our technical support staff can provide advice concerning installation and
operation of ADI products. Services outside of this may incur a charge. Technical support
staff will not provide experimental protocols or procedural instructions for conducting
experiments. However, information of this type may be provided in the supplied product
documentation, or on ADI web sites.
Inquiries
For additional information or service inquiries please contact the nearest ADInstruments
office or Authorized Distributor. For contact details see www.ADInstruments.com
C006E
Copyright © ADInstruments NZ Ltd, 2020. All rights reserved. PowerLab, LabChart and ADInstruments are
registered trademarks of ADInstruments NZ Ltd. Windows 8, Windows 7, Windows Vista and .NET Framework
are trademarks of Microsoft Corporation. Apple, the Apple logo, MacOS, and Macintosh are trademarks of
Apple Computer Inc. registered in the U.S. and other countries. Acrobat and Adobe are registered trademarks of
Adobe Systems Incorporated. Igor is a trademark of Wavemetrics Inc. MATLAB is a registered trademark of The
MathWorks Inc. Grass is a trademark of Astro-Med Inc. All other trademarks are the property of their respective
owners.

6479-D

adinstruments.com
6479
-
A
Other manuals for Human NIBP Nano
1
Table of contents
Other ADInstruments Medical Equipment manuals