Atmos C 051 Thorax User manual

E349855
0124
GA1GB.710101.0
2021-04 Index: 16
These operating instructions are valid from software version 1.3.22.
Operating instructions
ATMOS C 051 Thorax
English

2
Table of contents
1.0 Introduction...................................................................................................... 4
1.1 Notes on operating instructions .............................................................................4
1.2 Intended use..............................................................................................................5
1.2.1 Intended use ATMOS C 051 Thorax ........................................................................5
1.2.2 Intendedusesecretioncanister800ml.................................................................6
1.2.3 Intendedusehosesystem.......................................................................................7
1.3 Function......................................................................................................................9
1.4 Transport and storage..............................................................................................9
1.5 Explanationofpicturesandsymbols ...................................................................10
2.0 For your safety ............................................................................................... 14
2.1 General safety instructions....................................................................................14
2.2 Danger for users, patients and third parties .......................................................14
2.3 Avoidingdamagetothedevice .............................................................................17
2.3.1 Generalinformation ...............................................................................................17
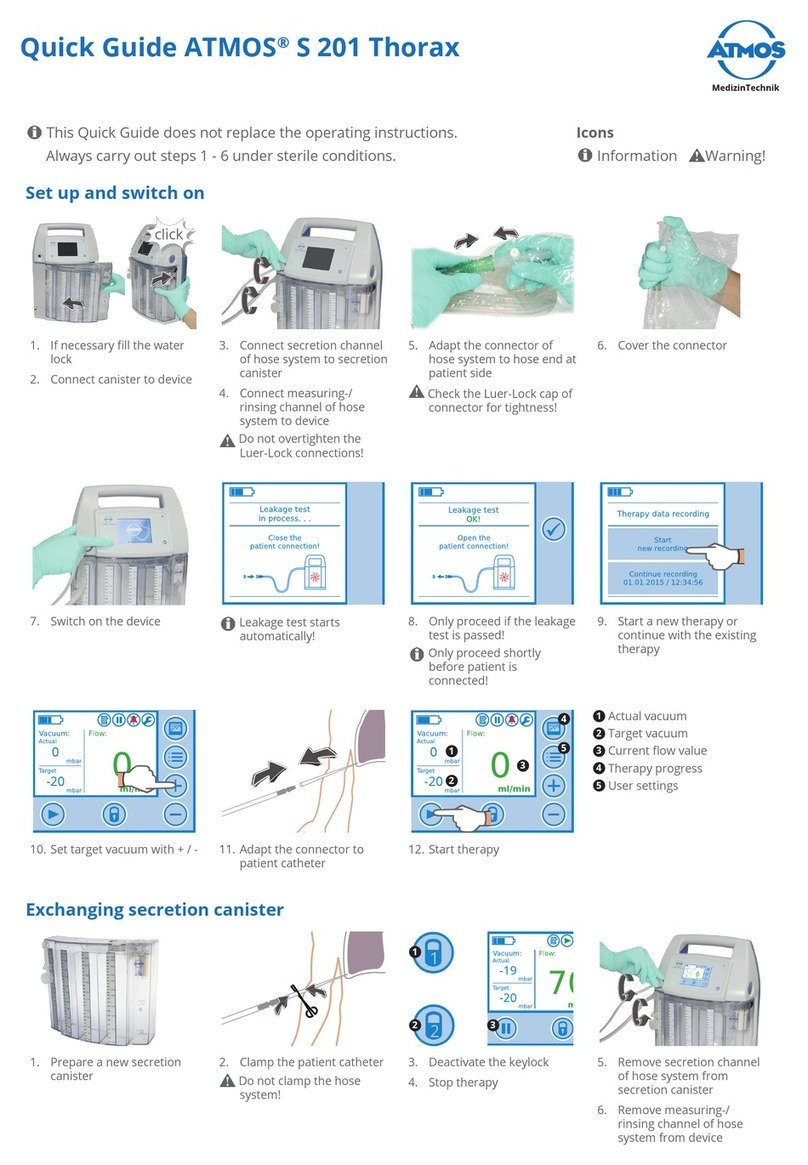
3.0 Setting up and starting up............................................................................ 19
3.1 Scope of delivery.....................................................................................................19
3.2 Device overview.......................................................................................................19
3.3 Start up.....................................................................................................................20
3.3.1 Battery charging ......................................................................................................20
3.3.2 Secretion canister....................................................................................................21
3.3.3 Connectthehosesystem.......................................................................................24
4.0 Operation........................................................................................................ 26
4.1 Explanation of the display......................................................................................26
4.2 Buttonsanddisplaysymbols.................................................................................27
4.2.1 Buttons.....................................................................................................................27
4.2.2 Displaysymbols.......................................................................................................28
4.3 Explanationofthedisplayinkeylockmodes......................................................28
4.3.1 Keylockmodewithbubbles..................................................................................28
4.3.2 Keylockmodewithbarchart................................................................................28
4.4 Switching on.............................................................................................................29
4.5 Leakage test.............................................................................................................29
4.6 Function....................................................................................................................31
4.6.1 Targetvacuum .........................................................................................................31
4.6.2 Gravitydrainagemode...........................................................................................31
4.6.3 Suction......................................................................................................................31
4.7 Key lock.....................................................................................................................33
4.8 Therapy process ......................................................................................................34
4.8.1 Shorttimedisplay: ..................................................................................................34
4.8.2 Longtimedisplay: ...................................................................................................35
4.8.3 Transfer of therapy data.........................................................................................36
4.8.4 Reading out the therapy data................................................................................37
4.9 User settings............................................................................................................38
4.10 Switchingothedevice..........................................................................................40
5.0 Warning messages ......................................................................................... 41
6.0 Function .......................................................................................................... 44
6.1 Hose rinsing.............................................................................................................44
6.2 Gravitydrainagemodewhileusingthedrainagesystem..................................44
7.0 Accessories, consumables and spare parts ................................................ 45
7.1 Attachingtheuniversalbracket(Accessories) .....................................................45
7.2 Attaching/removingthedeviceto/fromtheuniversalbracket..........................46
7.3 Attaching the support to a standard rail..............................................................47

7.3.1 Attach the support directly to a standard rail......................................................47
7.3.2 Attaching/removingthesupportto/fromtheuniversalbracket.......................47
7.4 Placing/removingthedeviceon/fromsupport(accessory) ............................48
7.5 Attachingandremovingthepowersupplyunit(accessory)..............................48
7.5.1 Attachingandremovingthepowersupplyunittothesupport........................48
7.5.2 Attachingandremovingthepowersupplyunitwithsupporttotheuniversal
bracket......................................................................................................................49
7.6 Insertingandremovingthepowersupplyunitandpowercable .....................49
7.7 Chargingthedevicewithpowersupplyunitsupport(accessory) ....................49
7.8 Attachingandremovingthecarryinghandle,disposablestrapandcarrying
strap..........................................................................................................................50
7.8.1 Strap holders ...........................................................................................................50
7.8.2 Attaching the carrying handle ...............................................................................50
7.8.3 Removingthecarryinghandle...............................................................................51
7.8.4 Attachingthedisposablestrap..............................................................................51
7.8.5 Attaching the carrying strap ..................................................................................51
8.0 Cleaning and care .......................................................................................... 53
8.1 Generalinformationoncleaninganddisinfection .............................................53
8.2 Cleaning the device surface...................................................................................54
8.3 Recommendeddisinfectants.................................................................................54
8.4 Hygiene plan............................................................................................................55
9.0 Maintenance and service.............................................................................. 56
9.1 Basic instructions ....................................................................................................56
9.2 Repairs......................................................................................................................56
9.3 Sending in the device..............................................................................................56
9.4 Handlingofbatteries..............................................................................................57
10.0 Troubleshooting ............................................................................................. 58
11.0 Technical data ................................................................................................ 60
11.1 Bacterialandvirallter ..........................................................................................61
12.0 Disposal........................................................................................................... 62
13.0 Notes on EMC (Electromagnetic compatibility).......................................... 63
13.1 Guidanceandmanufacturer’sdeclaration–ambientconditions.....................63
13.2 Guidanceandmanufacturer’sdeclaration–keyfeatures .................................63
13.3 Guidanceandmanufacturer’sdeclaration–warnings.......................................63

4
1.0 Introduction
1.1 Notes on operating instructions
Theseoperatinginstructionsarevalidfromsoftwareversion
1.3.22.
Theseoperatinginstructionscontainimportantnoteson
how to operate the ATMOS C 051 Thorax safely, correctly and
eectively.
Their reading helps to avoid risks, and also to reduce repair
costsanddown-times.Thisincreases,amongotherthings,
thereliabilityandservicelifeofthedevice.
These operating instructions serve not only for new operating
personneltobeinstructedinitsuse,butalsoforuseasa
referencemanual.Thisdocumentmayonlybereprinted,
eitherinpartorinwhole,withwrittenpermissionfrom
ATMOS.
These operating instructions must always be kept
available near the device.
Care and periodic tests in conjunction with professional
execution provide for operational safety and readiness for
useofyourATMOSC051Thoraxandarethereforeamust
besidesregularcleaning.
Repairworkandperiodictestsmaybecarriedoutonly
byexpertpersonnelauthorisedbyATMOS.Byapplying
only original spare parts you will have the guarantee that
operational safety, readiness for work and the value of your
ATMOSC051Thoraxwillbepreserved.
§
• ThisdevicebearstheCEmarkingCE0124inaccordance
withtheEuropeanMedicalDeviceRegulation(MDR)
2017/745.
• TheproductATMOSC051Thoraxcomplieswithall
applicablerequirementsofthedirective2011/65/EC
restrictingtheuseofcertainhazardoussubstancesin
electricalandelectronicequipment(“RoHS”).
• TheDeclarationsofConformityandourGeneralTerms
andConditionscanbeviewedonourwebsiteatwww.
atmosmed.com.
• ThequalitymanagementsystemappliedatATMOShas
beencertiedaccordingtointernationalstandardsENISO
13485.
• Prior to starting up please peruse chapter “2.0Foryour
safety”onpage14,inordertobepreparedforany
possibledangeroussituations.

5
1.2 Intended use
1.2.1 Intended use ATMOS C 051 Thorax
Name: ATMOS C 051 Thorax
Main function: TheATMOSC051Thoraxisadeviceformobile,digital
cardiothoracicdrainage.Thesystemgeneratesa
controlledvacuumclosetothepatientandhasan
electronicmonitoringsystemwhichshowsthecurrent
vacuummeasuredatthepatient'ssideandtheairleak.
Theobjectivetherapydataaredisplayedinrealtimeand
illustratedincolourinagraph.Errorsareautomatically
indicatedbyvisualandacousticwarningmessages.
Intended use / intended
purpose:
Restoringthe(natural)vacuuminthepleuralcavityby
drainingoairandsecretion.
Intended users / user prole: • Trained doctors
• Trained healthcare professionals
Users must not be hard of hearing or deaf and must have
adequate vision.
Intended patient group: Patients of all ages with and without restrictions.
Medical condition to be
diagnosed, treated or
monitored:
Accumulatedairanduidsinthethorax(pleuralcavity,
mediastinum,pericardium),whichmustbedrained,
monitoredandbalancedinacontrolledmanner.
Application organ: Thorax(pleuralcavity.mediastinum,pericardium).
Application time: Short-termuseonthepatient(<30days)
Area of application: The application site is the clinical area. The cardiothoracic
drainagesystemmayonlybeappliedbyhealthcare
professionals. The device is applied unsterile. The
secretioncanisterandthehosesystemaresterileand
disposable;theycanbeusedinthesterileOTarea.
Criteria for patient selection: Patientswhorequireacardiothoracicdrainage(pleura,
mediastinalorpericardialdrainage).
Indications: • After surgical openings of the thorax
• Pneumothorax
• Pleuraleusion
• Hemothorax
• Pleuralempyema
• Chylothorax
• Othersimilarclinicalpictures
Medical contraindications: • Notsuitableforuseonpatientswithlargeairleaks(≥4,5
l/min)andcoagulum.
• Notsuitableforcardiothoracicdrainagetherapyin
whichnovacuumshouldbeappliedtothepatient.
Other contraindications: • No separate application of the secretion canister and
thehosesystem(i.e.withoutdevice)asgravitydrainage.
• Noapplicationunderemergencyconditions.
• Shouldnotbeusedinthehomecaresectorwhichisnot
supervisedbyhealthcareprofessionals.
• Nodrainageofammable,corrosiveorexplosiveuids/
gases.

6
Warning notes: Thefollowingcomplicationsmayoccurduringa
cardiothoracic drainage:
• Pain due to irritation of the intercostal nerves
• Injuriestothelungparenchyma/airleakage
• Re-expansionedema
• Eusionretention
• Tensionpneumothorax
• Skin/softtissueemphysema
The product is: xactive qnot active
Sterility / specic microbial
condition:
• Notrequiredforthedevice.
• Secretioncanisterandhosesystemaresterile.
Single-use product /
reprocessing:
• Options for reprocessing the device according to the
operating instructions.
• Secretioncanisterandhosesystemaredisposable.
Fordetailedinformationonthesecretioncanisterandhosesystem,pleaserefertotheseparate
intended uses.
1.2.2 Intended use secretion canister 800ml
Name: Secretioncanister800ml
Main function: Thesecretioncanistertransfersthecontrolledvacuum
generatedbytheATMOSC051Thorax.Fluidsandairare
drained through the secretion hose and collected in the
secretioncanister.Theamountofuidinthesecretion
canistercanbereadanddocumentedonthebalancing
scales.Anintegratedbacterialandvirallterprotectsthe
devicefrompossiblecontaminationandfromoversuction.
Asaprotection,thepop-ovalveopenswhenthereis
excess pressure in the secretion canister.
Cover caps are used for proper sealing and disposal.
Intended use / intended
purpose:
Collectionofuidsandairfromthethorax.
Balancingtheamountofuid
Intended users / user prole: • Trained doctors
• Trained healthcare professionals
Users must not be hard of hearing or deaf and must have
adequate vision.
Intended patient group: Patients of all ages with and without restrictions.
Medical condition to be
diagnosed, treated or
monitored:
Accumulatedairanduidsinthethorax(pleuralcavity,
mediastinum,pericardium)thatrequirecontrolled
drainage,monitoring,andbalancing.
Application organ: Thorax(pleuralcavity.mediastinum,pericardium).
Application time: Short-termuseonthepatient(<30days)
Area of application: The application site is the clinical area. The cardiothoracic
drainagesystemmayonlybeappliedbyhealthcare
professionals.Thesecretioncanisterandthehosesystem
aresterileanddisposable;theycanbeusedinthesterile
OT area.
Criteria for patient selection: Patientswhorequireacardiothoracicdrainage(pleura,
mediastinalorpericardialdrainage).

7
Indications: • After surgical openings of the thorax
• Pneumothorax
• Pleuraleusion
• Hemothorax
• Pleuralempyema
• Chylothorax
• Othersimilarclinicalpictures
Medical contraindications: • Notsuitableforuseonpatientswithlargeairleaks(≥4,5
l/min)andcoagulum.
• Notsuitableforcardiothoracicdrainagetherapyin
whichnovacuumshouldbeappliedtothepatient.
Other contraindications: • Do not use with cardiothoracic drainage devices other
than the ATMOS C 051Thorax
• No separate application of the secretion canister and
thehosesystem(i.e.withoutbasicdevice)asgravity
drainage.
• Noapplicationunderemergencyconditions.
• Shouldnotbeusedinthehomecaresectorwhichisnot
supervisedbyhealthcareprofessionals.
• Nosuctionofammable,corrosiveorexplosiveuids/
gases
Warning notes: Thefollowingcomplicationsmayoccurduringa
cardiothoracic drainage:
• Pain due to irritation of the intercostal nerves
• Injuriestothelungparenchyma/airleakage
• Re-expansionedema
• Eusionretention
• Tensionpneumothorax
• Skin/softtissueemphysema.
The product is: qactive xnot active
Sterility / specic microbial
condition:
Secretion canister is sterile.
Single-use product /
reprocessing:
Secretioncanisterisdisposable.
1.2.3 Intended use hose system
Name: Hosesystem
Main function: Thedoublelumenhosesystemtransfersthecontrolled
vacuumgeneratedbytheATMOSC051Thorax.The
secretionhosedrainsuidsandairintothesecretion
canister.Themeasuringandrinsinghosemeasuresand
regulatesthevacuumappliedonthepatient’sside.A
bacterialandvirallteronthemeasuringandrinsing
hoseprotectsagainstcontaminationwithbacteriaand
viruses.Atdenedtimeintervals,avalveopensinorderto
directairthroughthemeasuringandrinsinghoseintothe
secretionhoseandtorinseliquids,coagulumandother
blockagesintothesecretioncanister.
Chesttubesonthepatient'ssideareconnectedtothe
hosesystemviatheport.

8
Intended use / intended
purpose:
Transportofuidsandairfromthethorax.Measurement
andregulationofthevacuumonthepatient'sside.
Intended users / user prole: • Trained doctors
• Trained healthcare professionals
Users must not be hard of hearing or deaf and must have
adequate vision.
Intended patient group: Patients of all ages with and without restrictions.
Medical condition to be
diagnosed, treated or
monitored:
Accumulatedairanduidsinthethorax(pleuralcavity,
mediastinum,pericardium),whichmustbedrained,
monitoredandbalancedinacontrolledmanner.
Application organ: Thorax(pleuralcavity.mediastinum,pericardium).
Application time: Short-termuseonthepatient(<30days)
Area of application: The application site is the clinical area. The cardiothoracic
drainagesystemmayonlybeappliedbyhealthcare
professionals. The device is applied unsterile. The
secretioncanisterandthehosesystemaresterileand
disposable;theycanbeusedinthesterileOTarea.
Criteria for patient selection: Patientswhorequireacardiothoracicdrainage(pleura,
mediastinalorpericardialdrainage).
Indications: • After surgical openings of the thorax
• Pneumothorax
• Pleuraleusion
• Hemothorax
• Pleuralempyema
• Chylothorax
• Othersimilarclinicalpictures
Medical contraindications: • Notsuitableforuseonpatientswithlargeairleaks(≥4.5
l/min)andcoagulum.
• Notsuitableforcardiothoracicdrainagetherapyin
whichnovacuumshouldbeappliedtothepatient.
Other contraindications: • No application with other cardiothoracic drainage
systemsotherthantheATMOSC051Thorax,ATMOSS
201 Thorax and ATMOS E 201 Thorax
• No separate application of the secretion canister and
thehosesystem(i.e.withoutdevice)asgravitydrainage.
• Noapplicationunderemergencyconditions.
• Shouldnotbeusedinthehomecaresectorwhichisnot
supervisedbyhealthcareprofessionals.
• Nosuctionofammable,corrosiveorexplosiveuids/
gases
Warning notes: Thefollowingcomplicationsmayoccurduringa
cardiothoracic drainage:
• Pain due to irritation of the intercostal nerves
• Injuriestothelungparenchyma/airleakage
• Re-expansionedema
• Eusionretention
• Tensionpneumothorax
• Skin/softtissueemphysema.

9
The product is: qactive xnot active
Sterility / specic microbial
condition:
Hosesystemissterile.
Single-use product /
reprocessing:
Hosesystemisadisposableproduct.
1.3 Function
TheATMOSC051Thoraxisanexceptionallyhandy,portable,digitalthoracicdrainagesuction
device.
Thedeviceisoperatedwithanelectrical,maintenance-freediaphragmpump.Duringoperation
thepumpcreatesavacuumwithinthesuctionhoseandthesecretioncanisterbymeansof
whichsecretionandaircanbesuckedobythehosesystem.Thepumpiscontrolleddigitally
andthereforeensuresthatthechosenrequiredvacuumvalueisstable.Theairow,which
ismeasuredinrealtime,isdisplayedinnumbers.Thesecretioniscollectedinthesecretion
canister.Itscapacityis800ml.Withtheaidofthemeasuringandrinsinghosethevacuumatthe
endofthehosesystemismeasured.Viathetouchscreendisplaythetargetvacuumcanbeset
manually.Thesuctioncapacityisregulatedautomatically.
Thehosesystemisrinsedwithairatregularintervalstopreventthecollectionofdebrisinthe
secretionhose.Thismeasurealsopreventssecretionfromintrudingintothemeasuringand
rinsinghoseorthatasyphoneectiscreated.
Thedeviceisequippedwitharechargeablebattery.Achargingunitwhichislocatedwithinthe
suctiondeviceguaranteesforthesecurechargingofthebattery.Thereforeitisimpossibleto
overchargethebattery.
Bacterialandviralltersinthesecretioncanisterandmeasuringhosepreventcontaminated
secretionsfromenteringthedevice.Thedeviceisequippedwithadisposablestrapanda
carryinghandle.Theseenablemobilityandmountingofthedevicee.g.tothepatientbed.A
universalbracketorthestandardrailbracketcanbeorderedseparatelyasaccessories.
1.4 Transport and storage
• Thedevicemayonlybetransportedinaupholsteredandprotectiveshippingbox.
• Pleasenotedownandimmediatelyreportanydamageswhichoccurredduringshipping.
PleasemakeuseoftheattachedQD434Deliverycomplaint/Returnshipmentformifyou
haveacomplaintorsendbackthedevice.Thisformcanalsobedownloadedfromourwebsite
www.atmosmed.com.
• Afterthetransportoftheunitintemperaturesbelow0°Corpriortorststartupitshouldbe
keptatroomtemperatureforatleastsixhours.Ifthedeviceisnotacclimatizeditmaynotbe
usedasdamagetothediaphragmsofthepumpcouldoccur.
• Ambientconditions:
Transport / storage: −20...+50°C;
5...95%airhumiditywithoutcondensationatairpressure700...1060
hPa
Operation and
batterycharging:
+5...+35°C;
20...80%airhumiditywithoutcondensationatairpressure700...1060
hPa
Operating altitude: max.3000m

10
1.5 Explanation of pictures and symbols
In the operating instructions
DANGER
Warningofadangerthatwillresultinimmediatefatalorseriousinjury.Observethenecessary
measures.
WARNING
Warningofadangerthatcancausefatalorseriousinjury.Observethenecessarymeasures.
CAUTION
Warningofadangerthatcancauseminorinjury.Observethenecessarymeasures.
NOTICE
Noticeofadangerthatcandamagetheproductorotherobjects.Observethenecessary
measures.
Warningofadangerthatcancausefatalorseriousinjury.
i
Noticeofpotentialmaterialdamage.
Usefulinformationonthehandlingofthedevice.
1. Action.Proceedstepbystep.
»Result of an action.
·Generalinformation,numeration
-Sub-numeration
click Engage,checkcorrectt.
Follow the arrows
Move in this direction, plug in.
Please press where dot indicates
Activate the optional foot switch
Replace
Check

11
Symbols used for the ATMOS C 051 Thorax and its accessories
Medical device
ThisdevicecomplieswiththerelevantrequirementsofEUregulations.
0124
ThisdevicecomplieswiththerelevantrequirementsofEUregulations.
E349855
UL Mark
MEDICALEQUIPMENT
withrespecttoelectricalshock,re,and
mechanicalhazardsonlyinaccordancewith
UL60601-1/ANSI/AAMIES60601-1(2005)/
CAN/CSA–C22.2No.60601-1(2008)
ThisdevicecomplieswiththerelevantrequirementsoftheEurasianEconomic
Union.
GOSTCerticate(Russia)
UniqueDeviceIdentierofamedicaldevice
REF
Articlenumber
SN
Serialnumber
LOT
Batch code
Use-bydate
Manufacturer
Dateofmanufacture
Countryofmanufacture:Germany
Warning,payspecialattention
Observetheoperatinginstructions!
Followoperatinginstructions(blue)

12
Do not reuse
STERILE
Sterile device
STERILE EO Sterilized using ethylene oxide
NON
STERILE Non-sterile
Doublesterilebarriersystem
Singlesterilebarriersystem
Protection class II device
Applied part type BF
IP 33 Specicationofthedegreeofprotectionagainsttheingressofsolidsand
moisture
Fuse
Donotuseifthepackagingisdamagedandfollowtheoperatinginstructions.
Fragile, handle with care
Keep dry
Keepawayfromsunlight
Temperaturelimit
Atmosphericpressurelimitation
Humiditylimitation

13
UDI application identier
(01) UDI-DI:Identicationofthemanufacturerandthedevice
(10) Batch code
(11) Dateofmanufacture
(17) Expiry date
(21) Serialnumber

14
2.0 For your safety
ThesafetyoftheATMOSC051Thoraxcomplieswithalltherecognizedrulesoftechnologyand
the guidelines of the Medical Devices Act.
2.1 General safety instructions
Familiariseyourselfwiththedeviceingoodtimesothatyouarecapableofoperatingitatany
time.
Neveroperatethedeviceifitshowsanyobvioussafetydefects.
Onlyafullyfunctionalproductmeetsthesafetyrequirementsofusers,patientsandthird
parties.Therefore,pleaseobservethefollowinginstructionsonyourproduct:
2.2 Danger for users, patients and third parties
WARNING
Electric shock due to unsuitable mains power connection, incorrect handling of the
product or damage to product components.
Burns,cardiacarrhythmiasandevenfatalinjuryarepossible.
• Donotoperatethedeviceifithasbeendropped.Inthiscase,cleananddisinfectthedevice
and send it to ATMOS for repair.
• Ifthedevicehasfallen:Checkthedeviceforvisibledamage.Aleakagetestisrecommended.
Iftheleakagetestfailsorthehousingisdamaged,thedeviceisdefectiveandmustnotbe
operated. In this case, clean and disinfect the device and send it to ATMOS for repair.
• Priortoeachuse,checkfordamagetothedeviceandthepowercable.Donotoperatethe
deviceifyounoticeanydamage.Inthiscase,cleananddisinfectthedeviceandsenditto
ATMOS for repair.
• Damagedcablesmustbereplaced.
• Youcanonlydisconnectthedevicefromthepowersupplybypullingoutthepowerplug.
• Positionthedeviceinsuchawaythatyoucaneasilydisconnectitfromthemainspower
supplyatanytime.
• Whendisconnectingthedevicefromthemainspowersupply,pullthepowerplugrstand
then the device plug.
• Disconnectthedevicefromthemainspowersupplybeforecleaningordisinfectingit.
• Nevertouchthepowerplugorpowercablewithwethands.
• Neverimmersethedeviceinwaterorotherliquids.
• Donottakeashower/bathwiththedevice.
• Thedeviceisnotsterilisable.
• Usethepowercableonlyindrysurroundings.Thesurroundingsmustbenon-conductive.
• Donotallowliquids(suchasdisinfectantsorsecretions)toenterthedeviceorpowersupply
unit unit.
• Ensurethatnoliquidentersthedevice.Ifliquidgetsintothedevice,operationofthedevice
mustceaseimmediately.Inthiscase,cleananddisinfectthedeviceandsendittoATMOSfor
repair or an authorised service partner.
• Ifdisinfectanthaspenetratedthedevice,thenitmustbedriedthoroughlyandsubsequently
aneciencycontrolmustbeconducted.Acheckshouldbecarriedouttoseewhether
thetargetvacuumisreachedwhenthesystemisclosedandwhetheraow>4l/minis
establishedafterawhilewhenthesystemisopen.Ifnot,itmustnotbeoperateduntilithas
beencheckedbyanauthorisedservicepartnerorATMOSServicecentre.
• UseonlyoriginalaccessoriesandoriginalsparepartsfromATMOS.Thisappliestothepower
cableinparticular.
• Follow the instructions on periodic tests in chapter “9.0Maintenanceandservice”onpage
56.

15
• Assembly,newsettings,modications,extensions,andrepairsmayonlybecarriedoutby
authorised persons.
• Donotmodifythedevicewithoutthemanufacturer’spermission.
WARNING
Risk of infection from non-sterile products!
Deadlydiseasescanbetransmitted.
• Neverusecomponentsmarkedwith morethanonce.Thesecomponentsareintendedfor
single use only.
• Repeatedreuseofcomponentswhicharemarkedwitha isforbidden.Thisproductisnot
re-sterilisable.Incaseofrepeatedreusethesecomponentslosetheirfunctionandthereisa
high infection risk.
• Onlyusesterilepackagedpartswhenthepackagingisundamaged.
• Priortouse,checkthepackagingofthesterileproducts,thesecretioncanistersystem,and
thehosesystemforintactness.Donotusedefectivesecretioncanistersorhosesystems.
• Repeatedreuseofsecretioncanistersandhosesystemscanleadtoinfections.
• Secretioncanistersandhosesystemshouldonlybeusedonceoneverypatient.
• Forhygienicreasonswerecommendanexchangeofbothsecretioncanisterandhosesystem
atthesametime.
WARNING
Risk of infection due to patient secretion on the device!
Deadlydiseasescanbetransmitted.
• Alwaysweardisposableglovesifyoucouldcomeintocontactwithsecretion.
• Clean and disinfect the device after every use.
• Clean and disinfect the device according to the operating instructions.
• Thedevicemustnotbeusedfollowingoversuction.
WARNING
Ensure that the device is always functional and ready for use!
Yourpatientcanbeseverelyinjured.
• Ensure that the device is always ready for use.
• Placethedevicewhereitiseasilyaccessible.
• Performafunctioncheckaftereachuse.
• ATMOSrecommendsalwayshavinganothersuctiondevicereadyathand.Thisallowsyouto
treatthepatientandperformsuctioningevenintheeventofdevicefailure.
• Pleaseobservethenotesontheelectromagneticcompatibility(EMC)ofthedevice.
• Ifthedevicehasfallen:Checkthedeviceforvisibledamage.Aleakagetestisrecommended.
Iftheleakagetestfailsorthehousingisdamaged,thedeviceisdefectiveandmustnotbe
operated. In this case, clean and disinfect the device and send it to ATMOS for repair.
• Thedeviceandthesecretioncanistermustalwaysbeusedvertically.Ifthedeviceshouldtilt
itmustbeplaceduprightagaininordertoguaranteefaultlessoperation.Ifyouareunsure
whether the secretion canister works properly we advise you to replace the secretion canister
toensurethepatients’safety.
• Thedeviceshouldnotbecarriedbythehosesystem.
• Priortoeveryuse,thedeviceshouldbecheckedforleakagesatthestartoftherapy(see
chapter “4.5Leakagetest”onpage29).Leakingconnectionscouldleadtoawrong
evaluationofthepatient’sstatusandcouldprolongthetherapy.Thusdocheckallconnections
for leakages to prevent the intrusion of additional air.
• Theleakagetestisrecommendedtocheckforleakagespriortoeachapplication.
• Thewarningmessage‘Deviceincriticaltilt’servesaspreventiveinformationtoavoid
malfunctioncausedbythedevicetiltingover(forexample,ablockedbacterialandvirallterin
thesecretioncanister).

16
• Theleakagetestfunctionandthewarningmessage‘Deviceincriticaltilt’areactivatedinthe
factorysettings.Ifthesefunctionsarenotdesired,theycanbedeactivatedintheusersettings
(chapter“4.9Usersettings”onpage38).
• Minimalleakagescanindicatesmallleaksinthesystemortoirregularitiesinthecourseof
therapy.Thiscanbeexcludedbyclampingthepatientcatheterandasaresulttheowvalueis
reduced to zero. If not, check all the connections on the device, the connectors as well as the
Luerlockcapforleakage.Ifthereisstillonlyaminimalowvalueillustratedthenthereisan
internalleakageinthesystemwhichcannotberectiedbytheuser.Thiswillbecompensated
bythesystembutillustratedasaminimalowvalue.
• ThedevicemaynotbeoperatedinMRIscanners(magneticresonanceimaging).
• TheATMOSC051Thoraxisamedicaldevicewhichissubjecttospecialsafetyregulations.It
musttobesetupandputintooperationinaccordancewiththeEMCregulations.Portable
andmobileRFcommunicationdevices(mobilephones)mayaecttheperformanceofthe
device.
WARNING
Avoid improper use.
Yourpatientcanbeseverelyinjured.
• Amisplaceddrainagesystemandamisplacedchesttubecouldhinderthedrainageofairand
liquids.Acompleteblockingofthesystemduringthedrainageofliquidsandaircouldcausea
riseinpressureandthusleadtoatensionpneumothorax.
• Employthedeviceonlyaccordingtoitsintendeduse.
• Thedeviceisnotsuitableforuseonpatientswithlargestulasandcoagulum.Forthese
patients,ATMOSrecommendstheuseofadevicewithgreatersuctioncapacity(e.g.ATMOSS
201Thorax).
• Theproductmayonlybeusedbymedicallytrainedpersonswhohavebeeninstructedinthe
handlingofthemedicalsuctionsystem.
• Pleaseselectthevacuumaccordingtothepatientandtheapplication.
• Observethevalidguidelines.
• Observethenotesonhygieneandcleaning.
• Alwaysplacethedrainagesystematthesameheightasthepatientscatheterandcheckthe
patienthoseforanybendsorcloggingwhichcouldhinderthedrainageofliquidandair.
Neverplacethedrainagesystemontheoor.
• Respondimmediatelytowarningmessage"Secretioncanisterfullorhoseblocked"/"Vacuum
toolow".Priortoexchangingthesecretioncanisterthechesttubemustbeclampedsothata
continuousvacuumisalwaysavailableatthepatient.
• Iftheuidlevelinthesecretioncanisteristoohighitcouldcauseablockageandthusa
tensionpneumothorax.
• Checkthesecretioncanisteratregularintervalsandalwaysreplaceitwhenthemaximum
llinglevelisreachedtoensurethepatientssafety.
• Checkthehosesystematregularintervals.Observetheinstructionsissuedbytheattending
physician.
• Thebendingofthepatienthoseleadstoaninterruptionofthetherapyandincorrect
measurements.
• Thehosesystemmaynotbeclamped.Ideallyclampthechesttubewhenchangingthe
secretion canister.
• Priortotheremovalofthehoseconnectorthechesttubemustbeclamped.
• Faultyrespectivelydamagedcomponentsmustbereplacedimmediately.
• Asetvacuumover-50mbarmaycausepainandinjurytothepatient.Setavacuumofmore
than-50mbaronlyifclinicallynecessary.

17
WARNING
Explosion and re hazard.
Burnsandinjuriesarepossible.
• UseonlyoriginalaccessoriesandoriginalsparepartsfromATMOS.Thisappliestothepower
cableinparticular.
• Never operate the product in potentially explosive areas or in areas that are oxygenated.
• Explosion-hazardousareasmaybecausedbytheuseofammableanaesthetics,skin
cleansingproductsandskindisinfectants.Theambientconditionsspeciedinthetechnical
data(chapter“11.0Technicaldata”onpage60)mustbestrictlyobserved.
CAUTION
Contact may cause allergic reactions!
Yourpatientcanbeinjured.
• Thematerialsusedhavebeentestedfortheirtolerability.Inveryrarecases,contactwith
accessiblematerialsonthedeviceanditsaccessoriesmaycauseallergicreactions.This
applies in particular to contact injuries in conjunction with prolonged contact. If this occurs,
seekmedicalattentionimmediately.
CAUTION
Tripping hazard due to cables.
Injuriesandfracturesarepossible.
• Laythepowercableproperly.
2.3 Avoiding damage to the device
NOTICE
Damage to the device due to improper use!
Thedevicemaybecomedamaged.
• Ensurethatnoliquidentersthedevice.Ifliquidgetsintothedevice,operationofthedevice
mustceaseimmediately.Inthiscase,cleananddisinfectthedeviceandsendittoATMOSfor
repair or an authorised service partner.
• Alwaysplacethedeviceonrm,levelsurface.Thedevicemustalwaysbeinaverticalposition
when you use it.
• Useonlypowercableswhicharefullyfunctional.
2.3.1 General information
• Compliancewithpropersurgicalproceduresandtechniquesistheresponsibilityofthe
treatingphysician.Observetheinstructionsissuedbytheattendingphysician.
• Theuserisobligedtoregularlycheckthefunctionalityofthedrainagesystemduring
operation.
• Thecontrolpanelmustbeclearlyvisibleandaccessiblefortheoperator.
• Thesecretioncanistermaynotbeusedwithoutthedevice(gravitydrainage).
• Thedevicemayonlybeoperatedbyqualiedpersonnel.
• Theremovalofthesecretioncanisterfromthedeviceduringthetherapymayonlybe
performedbytrainedprofessionalswhoactinconformitywithguidelines.
• Areadytousesparedeviceincludingconsumablesmustalwaysbeavailable.
• Thedevicesupportsthetherapyofthepatientitisnotasubstituteforthedoctors‘diagnosis.
• Thepatientshouldbesupervisedconstantlyinaccordancewiththeinternalrulesofthe
hospital.

18
i
Electromagnetic compliance, - damage to the device!
Thedevicemaybecomedamaged.
• TheATMOSC051Thoraxfullycomplieswiththeelectromagneticimmunityrequirements
ofstandardIEC60601-1-2/EN60601-1-2„Electromagneticcompatibility-MedicalElectrical
Devices“.
i
Damage to the device due to improperly installed protective contact socket!
Thedevicemaybecomedamaged.
• The ATMOS C 051 Thorax is designed in accordance with IEC 60601-1/EN 60601-1 and with
protection class ll.
• Thedevicemayonlybeconnectedtoaproperlyinstalledprotectivecontactsocket.
• Priortorststartingup,comparethemainsvoltageofthedevice(seerearofthepower
supplyunit)withthelocalmainsvoltage.
i
Storage and operation in an unsuitable environment.
Theelectronicscanbecomedamaged.
• Pleaseobservetheambientconditionsfortransport,storageandoperation.
• Alwaysplacethedeviceonrm,levelground.Thedevicemustalwaysbeinaverticalposition
whenyouuseit.Otherwise,secretionsmayenterthedevice.
i
Damage to the device due to low temperatures!
Thedevicemaybecomedamaged.
• Aftertransportofthedeviceintemperaturesbelow0°Corpriortorststartupitshouldbe
keptatroomtemperatureforatleastsixhours.Ifthedeviceisnotacclimatizeditmaynotbe
usedasdamagetothediaphragmsofthepumpcouldoccur.
i
Damage to device due to heat build-up!
Thedevicemaybecomedamaged.
• Do not cover the device during suction.
• Keepthedeviceandthepowercableawayfromotherheatsources.
• Donotplacethedevicedirectlynexttootherdevicesasthismaycauseexcessiveheatingof
the device.
• Thedeviceandthesecretioncanistershouldnotbedriedinamicrowaveoven.
• Thepowercableandthedevicemustbekeptawayfromhotsurfaces.
• Thedevicemayonlybeoperatedatroomtemperatureandshouldnotbesubjectedtodirect
solar irradiation as this could lead to errors.
i
Exclusion of liability and warranty
If
• original ATMOS parts are not used,
• theadviceforuseintheseoperatinginstructionsisnotbeingobserved,
• improperuse,
• assembly,newsettings,alterations,extensionsandrepairshavebeencarriedoutby
personnelnotauthorizedbyATMOS.
i
Advice on disposal
• Dispose of wrappings accordingly.
• Attentionmustbepaidtoallhospitalprotocolsregardingdisposalandinfectioncontrol.

19
3.0 Setting up and starting up
3.1 Scope of delivery
TheATMOSC051Thoraxwassubjectedtoanextensivefunctionaltestandwascarefullypacked
prior to dispatch.
Onreceiptofthegoodspleasecheckthepackagingforanypossibledamageandcomparethe
contentsforcompleteness.(seebillofdelivery)
317.0000.0 ATMOS C 051 Thorax
1x Basic device
1x Carrying handle 317.0090.0
5xDisposablestrap 316.1200.0
1x Recharging unit 313.0089.0
1xPowercable,L=4m 008.0941.0
1x Operating instructions
1xQuickreferenceguide
3.2 Device overview
Front side - without carrying handle
1Touchscreen
(touch-sensitivedisplay)
2On-/Osensor
3Charging socket
4Releasebuttonof
secretion canister
5Light sensor
6Clips for the carrying strap
Front side - with carrying handle
1Touchscreen
(touch-sensitivedisplay)
2On-/Osensor
3Charging socket
4Releasebuttonof
secretion canister
5Light sensor
6Attachmentforcarrying
handle

20
Rear side
11
12 13
7Connection for secretion
canister
8Mount for strap holder
9Type plate
10 Secretion canister guide
11 Measuring and rinsing
hose connection
12 Coversticker(nofunction
foruser)
13 Connection for USB
ashdrive(therapydata
transfer)
OnlyusetheUSBconnectionforthetransferoftherapydata.Asoftwareupdatemayonlybe
performedbyATMOSoranauthorizedserviceperson.
3.3 Start up
• Removethedevicefromthepackaging.Checkwhetherthemainscurrentonthetypeplateof
thepowersupplyunitcorrespondstothemainspowersupply.
• Perusesafetyinformationinchapter“2.0Foryoursafety”onpage14 prior to starting up
thedeviceforthersttime.
• Thebatterymustbefullychargedpriortotherstuse.Chargingtimeapprox.2.5hours.
• Place the device on a safe and even surface.
• Pluginthepowercabletorechargethebattery.
• Aftertransportingthedeviceatlowtemperatures,keepthedeviceatroomtemperaturefor
atleastsixhoursbeforeinitialstart-up.Ifthedeviceisnotacclimatizeditmaynotbeusedas
damagetothediaphragmsofthepumpcouldoccur.
• Alwayshaveatleastonemoresecretioncanisterathandasthedevicecanonlybeoperated
withthespecicATMOSsecretioncanister.
3.3.1 Battery charging
Eachbarofthesymbol represents20%batterycharge.
Attention!PriortorststartupoftheATMOSC051Thorax,thebatterymustbefullycharged.
OnlythebatterypowersupplyunitsuppliedbyATMOSshouldbeused.Pleasenotethe
informationonhowtohandlerechargeablebatteriesinchapter“9.4Handlingofbatteries”on
page 57.Correcthandlingoftherechargeablebatteriesprolongsthemaximumservicelife.
Batteriesarewearingpartsandthereforeexcludedfromthegeneralwarranty.Thedeviceshould
berechargedinacoolplacewithoutdirectsolarirradiation.Atambienttemperaturesabove
25°Cthechargingtimecouldbeprolongeddrastically.Defectswhichoccurduetoimproper
handlingofthedevicearenotcoveredbytheguarantee.
Attention:Thebatterycannolongerbechargedifthebatterytemperatureisabove35°C.
Other manuals for C 051 Thorax
4
Table of contents
Other Atmos Medical Equipment manuals

Atmos
Atmos E 341 User manual

Atmos
Atmos A 161 Battery Series User manual
Atmos
Atmos S 201 Thorax User manual

Atmos
Atmos C 451 User manual
Atmos
Atmos Chair M 2 User manual

Atmos
Atmos C 361 User manual
Atmos
Atmos C 051 Thorax User manual

Atmos
Atmos S 201 Thorax User manual

Atmos
Atmos Chair 41 Gyne User manual

Atmos
Atmos i View 21 User manual
Popular Medical Equipment manuals by other brands

Masimo
Masimo M-LNCS Series Instructions for use

C.T.S.V.
C.T.S.V. Medimachine II user manual

AliveCor
AliveCor Heart Monitor user manual

Game ready
Game ready ATX TRAUMATIC AMPUTEE Use guide

Aulisa
Aulisa Guardian Angel Rx GA2000 Instructions for use
Neurotech
Neurotech AvivaTens XP Instructions for use & warranty