Atmos A 161 Battery Series User manual

Gebrauchsanweisung
ATMOS®A / C 161 Battery
Operating instructions
English
GA1GB.310120.0
2019-04 Index: 32
0124

2
ATMOS
Phone: + 49 7653 689-0
Fax:
+ 49 7653 689-392 (Vertrieb Inland)
+ 49 7653 689-391 (Export)
e-mail: [email protected]
Internet: http://www.atmosmed.de
MedizinTechnik GmbH & Co. KG
Kudwig-Kegel-Str. 16
79853 Lenzkirch
Deutschland/Germany
Further information, accessories, consumables and
spare parts are available from:
Table of contents
8.0 Cleaning / Disinfection ......................................... 12-16
8.1 Basic information.......................................................... 12
8.1.1 Bacterial lter ............................................................... 12
8.1.2 Suction hose, hose connector and vacuum hose ........ 12
8.1.3 Fingertip ....................................................................... 12
8.1.4 Secretion canister ........................................................ 12
8.1.5 Canister lid ................................................................... 12
8.1.6 Device surface ............................................................ 13
8.1.7 Rinsing Canister........................................................... 13
8.1.8 Accessories.................................................................. 13
8.2 Oversuction ................................................................. 13
8.3 Cleaning instructions.................................................... 13
8.4 Recommended instrument disinfections ..................... 14
8.5 Recommended surface disinfections .......................... 14
8.6 Cleaning and servicing plan ........................................ 15
9.0 Maintenance and servicing ....................................... 17
9.1 Basic informationen...................................................... 17
9.2 Reprocessing ............................................................... 17
9.3 Battery handling ........................................................... 17
9.4 Sending in the device................................................... 18
10.0 Trouble-shooting........................................................ 19
11.0 Accessories, consumables, ...................................... 20
spare parts
11.1 Accessories.................................................................. 20
11.2 Consumables ............................................................... 20
11.3 Spare parts................................................................... 21
12.0 Technical specifi cations............................................ 22
13.0 Checking / Reprocessing / Disposal ........................ 23
13.1 Checking ATMOS suction devices ............................... 23
13.2 Reprocessing ............................................................... 23
13.3 Disposal ....................................................................... 23
14.0 Notes on EMC............................................................. 24
1.0 Introduction .................................................................. 3
1.1 Notes on operating instructions...................................... 3
1.2 Function ......................................................................... 4
1.3 Intended use .................................................................. 4
1.4 Scope of supply.............................................................. 5
1.5 Transport and storage ................................................... 5
1.6 Explanation of symbols ................................................. 5
2.0 Safety advice ................................................................ 6
3.0 Setting up and starting up........................................... 7
3.1 Operating elements....................................................... 7
3.2 Connection ..................................................................... 7
3.3 Starting up...................................................................... 7
3.4 Charging the battery....................................................... 7
3.4.1 Charging with battery charging power pack ................... 7
3.4.2 Charging with battery charger leads (12V)..................... 7
4.0 General operation ........................................................ 8
4.1 Suction hose .................................................................. 8
4.2 Adjust vacuum................................................................ 8
4.3 Suction procedure ......................................................... 8
4.4 Hose rinsing .................................................................. 8
5.0 Operation DDS.............................................................. 9
5.1 DDS canister and DDS bacterial lter ........................... 9
5.2 Insertion of the DDS canister ......................................... 9
5.3 Connect hose ................................................................. 9
6.0 Operation Receptal®................................................... 10
6.1 Holder for Receptal®secretion canister ....................... 10
6.2 Assembling the Receptal®secretion canister set........ 10
6.3 Connect hose ............................................................... 10
7.0 Operation Medi-Vac®and Serres®..............................11
7.1 Holder for Medi-Vac®secretion canister........................11
7.2 Assembling the Medi-Vac®secretion canister set ........ 11
7.3 Connect hose ............................................................... 11
7.4 Holder for Serres®secretion canister ............................11
7.5 Assembling the Serres®secretion canister set ............ 11
7.6 Connect hose ............................................................... 11
3
1.0 Introduction
1.1 Notes on operating instructions
These operating instructions contain important notes on how to operate the
ATMOS® A / C 161 Battery safely, correctly and e ectively. Their reading helps to avoid risks, and
also to reduce repair costs and down-time. That increases, amongst other things, the reliability and
service-life of the device.
These operating instructions serve not only for new operating personnel to be instructed in its use,
but also for use as a reference manual.
These operating instructions must always be kept available near the device.
Care and period tests in conjunction with professional execution provide for operational safety and
readiness for use of your ATMOS®A / C 161 Battery and are therefore a must besides regular clea-
ning.
Repair work and period tests may be carried out only by expert personnel authorised by ATMOS. By
applying only original spare parts you will have the guarantee that operational safety, readiness for
work and the value of your ATMOS®A / C 161 Battery will be preserved.
● The product ATMOS®A / C 161 Battery bears CE marking according to the EC Directive of the
council for medical products 93/42/EEC and meets the basic requirements of annex I of this
directive.
● The product ATMOS®A / C 161 Battery complies with all applicable requirements of the directive
2011/65/EC restricting the use of certain hazardous substances in electrical and electronic
equipment (“RoHS”).
● The declaration of conformity and our general standard terms and conditions can be obtained on
our website at www.atmosmed.com.
● The quality management system applied at ATMOS has been certi ed according to international
standard EN ISO 13485.
● Prior to start-up please peruse chapter 2.0 „For your safety“, in order to be prepared for any
possible dangerous situations.
●Reproduction of these instructions – even in part – only with the written permission of ATMOS.
●Subject to alterations and changes.
These operating instructions are valid for the following devices:
• ATMOS®A 161 Battery DDS... REF 313.0500.0
with 1 l DDS secretion canister
• ATMOS®C 161 Battery DDS. REF 313.0400.0
with 1 l DDS secretion canister
• ATMOS®C 161 Battery Receptal®REF 313.0465.0
with 1.5 l Receptal®canister
• ATMOS®C 161 Battery Medi-Vac®REF 313.0402.0
with 1 l Medi-Vac®canister
• ATMOS®C 161 Battery Serres®REF 313.0403.0
with 1 l Serres®canister
Please store this document near the device for later use!

4
1.2 Function
The ATMOS®A / C 161 Battery is a very handy small suction unit. It is
driven by an electromotive, maintenance-free swing piston pump. During
operation, the pump generates a vacuum within the hose system and the
collection canister, thus sucking o secretions or uids (e.g. by means
of a suction catheter). The uid is gathered in the collection canister. A
mechanical over ow safety (on the inner part of the collection canister
lid) avoids penetration of secretion into the pump head.
Valid for ATMOS®C 161 Battery
The nal vacuum and, following, the air- ow rate can be adjusted by me-
ans of the ne control and the vacuumgauge. An overtemperature stop
controlled by electronics avoids overheating of the unit.
Valid for ATMOS®A 161 Battery
The nal vacuum and, following, the air- ow rate can be adjusted by
means of 3 step vacuum regulation.An overtemperature stop controlled
by electronics avoids overheating of the unit.
For ATMOS®A / C 161 Battery / DDS:
The reusable secretion canister is connected to the pump housing via
direct-docking, without any pedestrian hose system. Only the suction
hose has to be plugged in by the user.
A bacterial lter, located in the lid of the secretion canister, avoids ente-
ring of bacterias and liquids into the pump interior. A mechanical over-
suction stop integrated in the canister lid additionally avoids accidentally
absorption of secretion into the pump head.
1.0 Introduction
Fig 1a.
ATMOS®C 161 Battery / DDS
Vacuum gauge
Vacuum control
On/o push button
Charge level indicator
Battery test button
Hose holder
Sliding cover for protecting the
control elements
Fig 1b.
ATMOS®A 161 Battery / DDS
Vacuum adjustment
On/o push button
Charge level indicator
Battery test button
Hose storage
1.3 Intended use
Name: ATMOS®A 161 Battery / ATMOS®C 161 Battery
Main functions: Temporarily and spontaneously suction of secretions,
blood and body fluids which typically accumulate during respiratory tract
suction and ENT treatment.
Med. indications/ application: Suction of the upper respiratory track
and throat, nose, ear
Specification of the main function: Drainage and temporarily collec-
tion of body fluids. By means of an electrical suction pump, a negative
pressure will be created. The integrated secretion canister allows a tem-
porarily collection of the derived body fluids.
Application organ: Upper respiratory track (oral cavity, nasopharyngeal
cavity and bronchial system) and throat, nose, ear
Application time: Temporary use on the patient during respiratory tract
suction (< 60 min.) or short-term use at ENT treatment (up to 30 days)
Application site: The application site of respiratory tract suction is the
clinical, practices, nursing and home care sector. In ENT medicine the
devices are used in clinic and practice.
The application of the device may only be performed by medical trained
and introduced staff.
Contraindications: Not adapted for:
• the continuous operation in case of drainages in the low vacuum range
(e.g. thoracic drainage or wound drainage).
• permanently endoscopic use.
• the use outside of the medical sector.
• the suction of flammable, corrosive and explosive substances.
• the suction in explosion-risk areas.
The product is: X active □ not active
Sterility: The device is not sterile.
Single use product / reprocessing: The device and part of the acces-
sories are reusable, for information on reprocessing and disinfection
please see the operating instructions.
!

5
1.4 Scope of supply
●Prior to dispatch, this ATMOS device was subjected to an extensive functional test and has been carefully packed.
Nevertheless, please compare the contents of the shipment on completeness immediately upon receipt (see delivery note).
In addition to the basic device, the scope of delivery comprises the following parts:
1.6 Explanation of symbols
The CE sign shows that this product
meets the appropriate requirements
of the EC guidelines.
1.5 Transport and storage
● The transport of the device may be e ected only in a dispatch
carton upholstered and o ering su cient protection.
● Please document and report damages in transit immediately.
For complaints or return deliveries, please use the enclosed
form QD 434.
●The unit must be allowed to stand for up to six hours at room
temperature prior to starting up for the rst time following
transport at temperatures below freezing. The unit may not
be operated if it has not acclimatised as this might damage its
diaphragms.
Warning, special diligent
notice!
Serial number
Order number
Application part type BF
Manufacturing date
Refer to operating
instructions.
Pump On / O
Battery test function
Fuse
Protection class II
Important information!
●Ambient conditions:
Transport/Storage: -30...+50° C;
5...90 % air humidity
non-condensing
at air pressure 700...1060 hPa
Operation and +10...+35° C;
battery charging: 20...80 % air humidity
non-condensing
at air pressure 700...1060 hPa
ATMOS®A / C 161 Battery, scope of supply of all versions:
Battery charging
power pack
Silicone
suction hose
Ø 6 mm, L= 1,30 m
1.0 Introduction
Grad. secretion
canister (1 l)
Lid for secretion
canister with
triple oversuction
safety
Medi-Vac®
canister (1 l)
Support
Receptal®
container (1.5 l)
Support
ATMOS®C 161 Battery
2 DDS
bacterial fi lter
ATMOS®A 161 Battery
1 DDS bacterial
fi lter
Medi-Vac®
suction bag (1 l)
with integrated
bacterial fi lter
Receptal®
suction bag (1.5 l)
with integrated
bacterial fi lter
Serres®
container (1 l)
Support
Serres®
suction bag (1 l)
Follow operating instruc-
tions! (blue)
Protection against the ingress of
- solid foreign objects Ø ≥ 12.5 mm
- vertically dripping water
Short term operation
Manufacturer
Eurasian conformity
Professional disposal
GOST Certi cate
(Russia)
REF
SN
DDS Receptal®
Medi-Vac®Serres®
i
IP 21
6
2.0 For your safety
Danger to the
device
● Do not allow any liquid to get into the unit. If
liquid has penetrated the unit, it may not be
operated again, until it has been checked by
the customer service centre.
● The unit must be set up on a rm, level sur-
face. The switched-on unit might get overheat-
ed if it is placed on an uneven surface (e.g.
mattress, cushion, padded seat etc.).
● The main voltage speci ed on the type plate
must match the power supply system.
●The unit may not be started:
•If cables or plugs are defective,
•If it has been dropped down before,
•If obvious defects might restrict safe
operation.
Clean the device prior to returning for repair.
●Pay attention to the ambient conditions de-
scribed in chapter 1.5 Transport and storage.
●The function of the device must be checked at
regular intervals for any safety related defects,
e.g. plug contacts, secretion canister, housing
etc.
●Never connect the unit to defective power
sockets or extension cables.
Avoid moisture on plug and switches.
●The unit, collection canister, mains cable, ac-
cessories, connection cables and hoses must
be checked for damage prior to starting up.
Damaged cables and hoses must be replaced
immediately. Prior to use, check the unit func-
tions.
Danger of injury!
● The ATMOS®A / C 161 Battery has been
designed for aspirating body uids in medical
ranges. Never remove explosive gases and
in ammable or corrosive uids.
● Only persons instructed in medical use may ap-
ply the ATMOS®A / C 161 Battery to patients.
● The unit may not be operated in splash water
range and in locations where there is a danger
of explosion.
General safety
information
● ATMOS cannot guarantee perfect functioning
neither will it be liable for damage to people
or property if:
• Any non-original ATMOS parts are
used,
• the user instructions given in this
manual are not followed exactly or
are disregarded,
• Assembly, resetting, alterations,
extensions and repairs are not carried
out by people authorised by ATMOS.
●Prior to starting up the ATMOS®A / C 161
Battery, read these operating instructions
carefully.
● No warranty rights shall exist in the event
of damage or failure caused by the use of
non-ATMOS®accessories or non-ATMOS®
consumables
● The safety standard of the ATMOS®A / C 161
Battery corresponds with recognized medical
technical regulations and the directions of the
law relating to medical products.
● Use transparent hoses only.
● Sterile packed parts may no longer be used
if their packing was damaged during trans-
port or storage.
Danger of infection for the patient.
●This suction unit may not be applied without
disposable bacterial lter plate.
●The suction hose must never come into direct
contact with the application site.
Always use a sterile suction catheter resp. a
medical accredited aspiration set.
● In order to disconnect the device from the
mains, please remove the plug of the battery
charging power pack from the wall socket.
● Prior to cleaning and servicing the device.
● Before emptying the secretion canister.
● Before leaving the room.
Never pull at the cable!
● During storage of the device continuous
recharging with the charging unit is required
in order to provide full performance in emer-
gency cases.
● In the case the pump is switched on, while a
high vacuum (> 40 %) is applied, the device
switches o automatically (safe condition). The
charge level indicator for the battery is blinking.
Switch on is only possible after ventilation of
the vacuum.
● This product is not re-sterilisable. Repeated
reuse of components which are marked with a
2is forbidden. In case of repeated reuse these
components lose their function and there is a high
infection risk.
● The ATMOS®A / C 161 Battery may be oper-
ated only in rooms used for medical purposes,
but not in areas subject to explosion hazards
and in oxygen rich environments.
● Please only use the provided medical power
supply unit (manufacturer: GlobTek Inc., model:
GTM91099-6015-3.0-T2).
!

7
3.0 Setting up
Set up the device on a level, rm surface.
3.1 Operating elements
3.2 Connection
The main voltage speci ed on the type plate must match
the power supply system.
Check mains cable for damages. Damaged cables must be
replaced immediately!
3.0 Setting up and starting up
3.3 Starting up
●The ATMOS®A / C 161 Battery is delivered ready for
use.
●Lift the unit out of the cardboard. Check whether the
voltage values on the data plate correspond with the
inbuilding voltage.
● Prior to rst use, the battery must be fully charged!
●Check whether the battery is fully charged, hereto
please press the battery test button (Figure 2, )!
When the battery is fully charged, all display elements
will light up (Figure 2,). If this is not the case, please
recharge the battery (see chapter 3.4).
●Set up the device on a level, rm surface.
●Prior to rst operation, pay attention to the safety
information in chapter 2.0.
●The unit must be allowed to stand for up to six hours at
room temperature prior to starting up for the rst time
following transport at temperatures below freezing. The
unit may not be operated if it has not acclimatised as
this might damage its diaphragms.
● ATMOS®A / C 161 Battery DDS: At least one DDS
bacterial filter must always be available because the
device may not be operated without filter!
Fig 3.
Fig 2.
Fig 4.
Fig 5.
3.4 Charging the battery
The battery is charged by the 12 V low voltage connector (Fig. 4).
Chapter 9.3 contains a reference to the battery handling.
3.4.1 Charging with battery charging
power pack
Connect the low voltage connector (Fig 4,) to the low voltage
socket at the device. Connect the mains plug of the battery
charging power pack (Fig 4,) to the wall socket.
3.4.2 Charging with battery charger leads
(12V)
Connect the low voltage connector to the cigarette lighter in
the car.
With both methods, full suction capacity is available during
charging. Even with fully discharged respectively defective
batteries it is possible to operate the device via 12 V low
voltage connection. The full suction capacity is available. In
order to prevent the device from fully discharge it switches o
automatically after approx. 10 minutes.
Battery capacity approx. 35 min.
Vacuum gauge (only for ATMOS®C 161 Battery)
Vacuum control
On/o push button
Charge level indicator
Battery test button
Hose holder
Sliding cover for protecting the
control elements (only for ATMOS®C 161 Battery)
Hose rewind (accessory)
Fig 4a.
i
i
C 161 Battery
high flow/
high vacuum
20 % 40 % 60 % 80 % 100 %
8
4.3 Suction procedure
●Then, lead in the catheter in the same way as shown by
your hospital sta and start the suction procedure.
●Control the suction procedure with the auxiliary air vent
(12) on the ngertip.
The hydrophobic DDS bacterial lter / oversuction stop
safely avoids ingress of moisture. Nevertheless you should
empty the canister at a ll level of 1/2.
●The secretion canister system is designed the way that
secretions ows laterally to the wall. Therefore the foam
formation in the canister is reduced.
●Only for ATMOS®A 161 Battery
Adjust your desired vacuum by turning the three-steps.
adjustment to the suitable position.
-25 kPa* low, -55 kPa*medium, -75 kPa* high
* depends on daily atmospheric pressure and
Ambient conditions
●Only for ATMOS®C 161 Battery
Adjust your desired vacuum by closing the suction
hose opening with the nger (11) the vacuum is then
generated. Open the regulating valve / vacuum control
(page 4, ) until the vacuumgauge shows the desired
vacuum value
●Choose a suction catheter of the right size (14 which
are available from ATMOS in 3 di erent sizes) or
a suction instrument which is only available from
specialized dealers.
4.0 General operation
4.2 Adjust vacuum
4.1 Suction hose
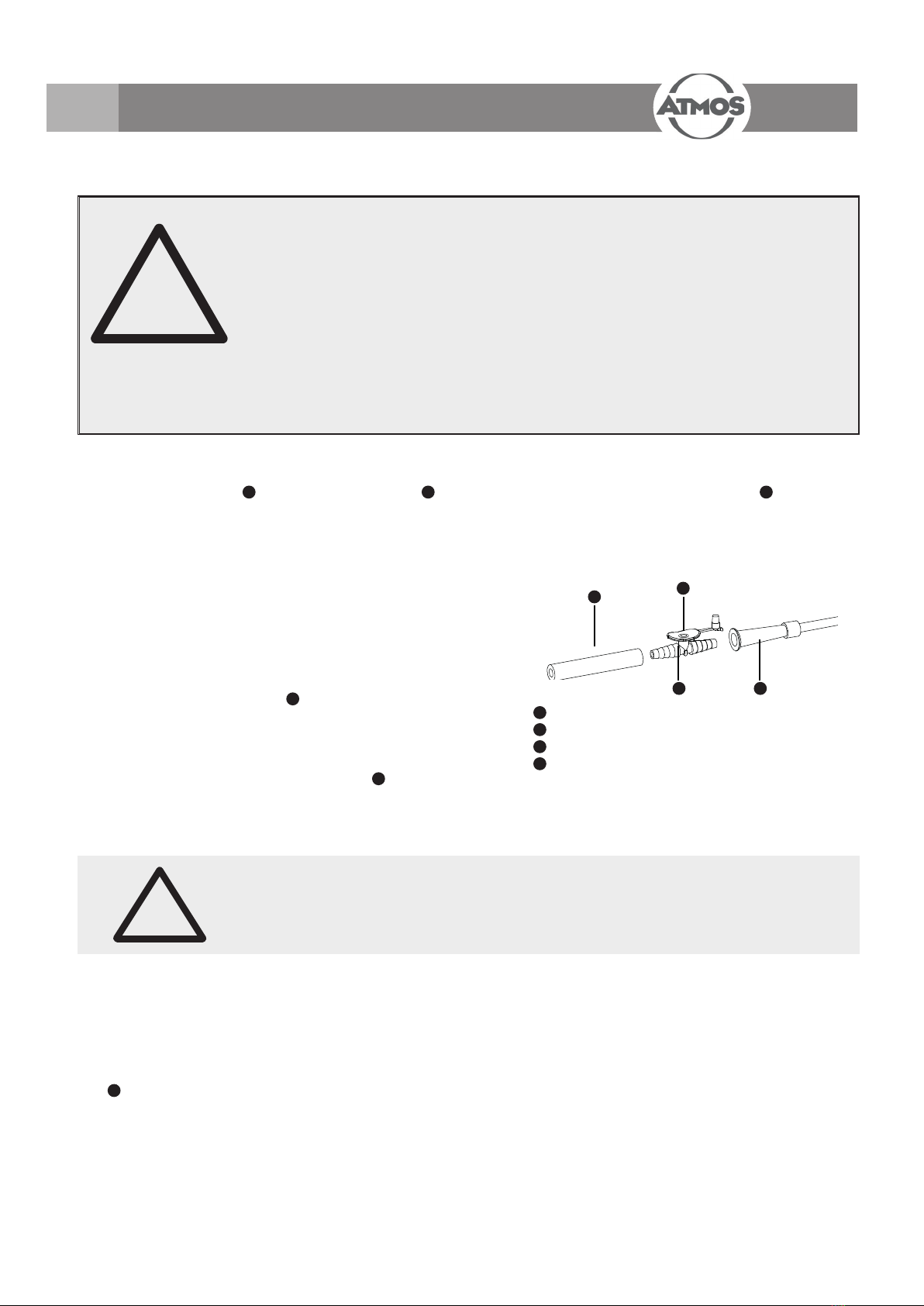
●Join the suction hose (11) and the suction catheter (14) by means of the ngertip (not included in delivery) (13).
4.4 Hose rinsing
●Dispose the suction catheter and rinse the
suction tube with clean water or disinfec-
tion liquid after every suction procedure. We
recommend to use the rinsing bottle in which
you can carry clean water along with you.
A separate rinsing canister set is available
(REF 313.0008.0).
Important
notes on safety
● Make sure that the collection canister
is evacuated in time. As soon as the
canister is half- lled, it must be emptied
(this principle proves right in all application
ranges).
●Attention: Suction procedures in the res-
piratory tract may only be implemented
after appropriate instruction by hospital or
special sta .
● When the maximum level is exceeded,
the over ow safety reacts and suction is
stopped. Empty the canister
● Check the vacuum readout regularly!
● If secretion has been soaked up into the
pump due to improper use or manipula-
tion, the device must be repaired by AT-
MOS or a service partner authorised by
ATMOS.
● For aspiration use only applicable suc-
tion catheters, attachments or a medical
aspiration sets.
● Whilst aspirating please pay attention to the
lling level of the secretion canister.
AUXILIARY AIR VENT OPEN =
suction procedure is interrupted (e.g. when leading in the catheter)
AUXILIARY AIR VENT CLOSED WITH THE FINGER = suction
Fig 6.
11 Suction hose
12 Auxiliary air vent
13 Finger tip
14 Suction catheter
Prior to these notes please read the foregoing chapter of your respective version of the ATMOS®A / C 161 Battery!
!
!
11
12
13 14
9
5.0 Operation DDS
Important notes on safety for the
DDS canister system
• Do not operate the device without a
bacterial fi lter. We recommend you
always to store at least one spare
bacterial fi lter.
• Always wear disposable gloves when
changing the bacterial lter!
• Prior to each use please check wheth-
er the bacterial lter is dry and clean.
Replace the bacterial lter with a new
lter if it is discoloured, contaminated
or oversucked. A bacterial lter may
never be reused.
• Exchange the bacterial lter when
changing the patient. ATMOS recom-
mends: Replace the bacterial lter after
14 days, even if there is no patient
change.
• Vacuum connection
Direct-Docking-System
The vacuum connection between the
pump and the collection canister is cre-
ated automatically as soon as the DDS
canister is positioned correctly.
5.1 DDS canister and
DDS bacterial fi lter
With the DDS collection canister on a rm surface, position
the lid horizontally on top (the lid may not be twisted!)
Press down lightly onto the collection canister using both
hands until limit is reached (Fig 7).
5.2 Insertion/Removal of the
DDS canister
The lter must be xed to the housing. Afterwards the
secretion canister is inserted. For insert the DDS collec-
tion canister, shift it horizontally onto the bacterial lter; for
removal, pull it horizontally outside (Fig 8).
Please note that this order is strictly observed otherwise
power loss of the device could be the consequence!
5.3 Connect hose
Press the required DDS hose adapter with 6 or 10 mm
diameter into the hole of the DDS collection canister lid
twisting slightly to ensure a tight t (Fig 10).
Twist slightly in the same manner when removing.
Fig 9.
Fig 8.
Fig 7.
i
Tip
Fig 10.
If required the canister can be ejected even easier from
the device by means of a lever instrument (e.g. plain
spattle); see Fig. 9.
!
!

10
6.0 Operation Receptal®
6.2 Assembling the Receptal®
secretion canister system
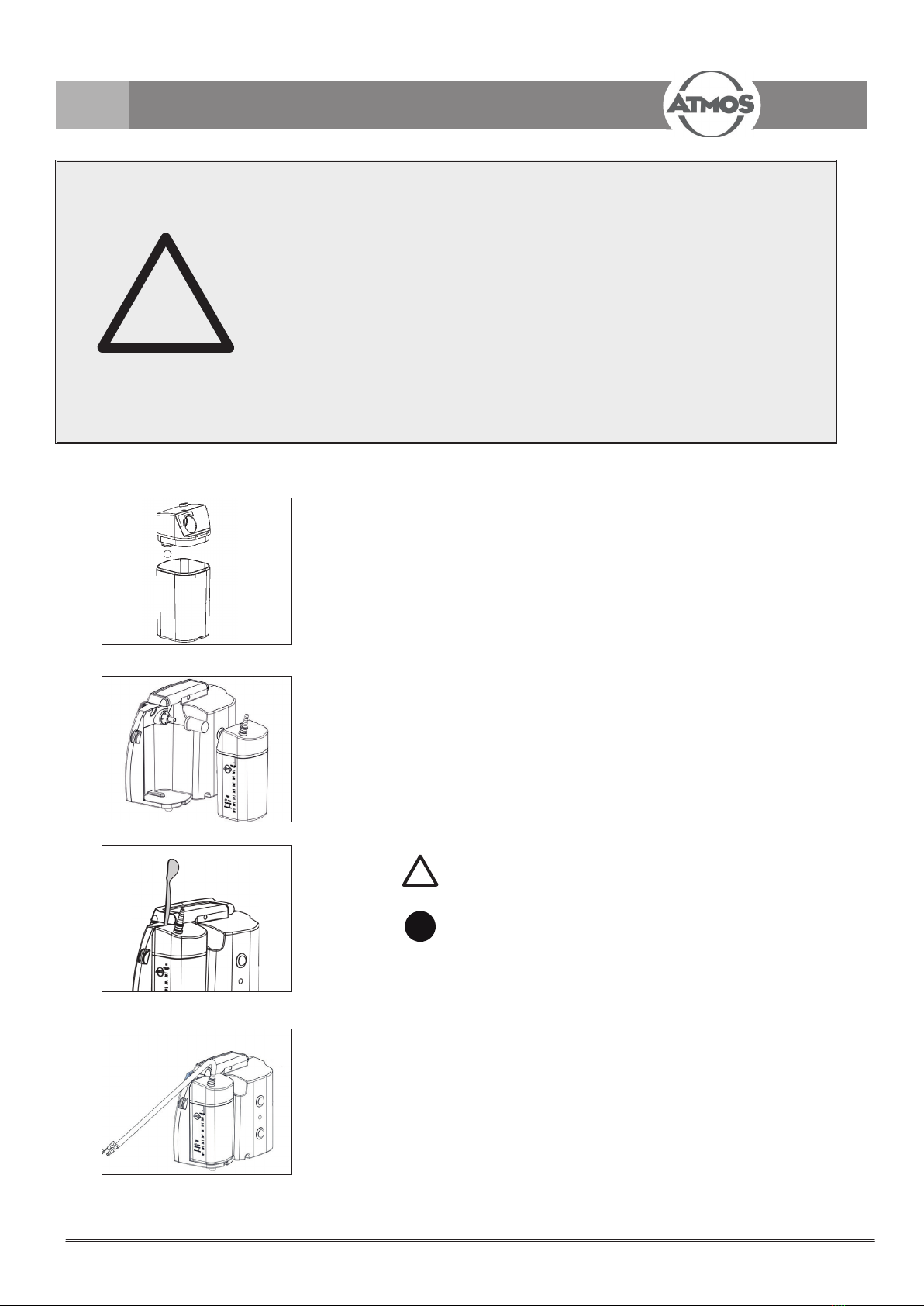
Insert the Receptal®bag into the Receptal®canister.
Close the canister tightly at all sides.
Check again for density, otherwise no vacuum can
be built up.
Insert the vacuum hose.
Only bags with integrated bacterial lter may be used!
Fig 12.
Fig. 13.
●Only use secretion bags with integrated
bacterial fi lter!
A bacterial fi lter avoids divulgement of
bacterias.
●Sterile packed parts may no longer be used if
their packing was damaged during transport or
storage
Danger of infection for the patient.
6.1 Holder for Receptal®secretion
canister
Plug the screw thread of the holder from the top into the
borehole at the bottom side of the device.
Screw it together with the provided screw nut.
Fig. 11.
Receptal®bag
Receptal®canister
15 Vacuum channel
16 Vacuum hose
17 Connection for vacuum hose
The secretion is sucked o through the vacuum
channel.
6.3 Connect hose
!
!
i
15 16 17
11
7.0 Operation Medi-Vac®and Serres®
7.1 Holder for Medi-Vac®secretion
canister
Plug the screw thread of the holder from the top into
the borehole at the bottom side of the device.
Screw it together with the provided screw nut.
Fig. 14.
7.2 Assembling the Medi-Vac®
secretion canister system
Fig.15.
Medi-Vac®bag
Medi-Vac®canister
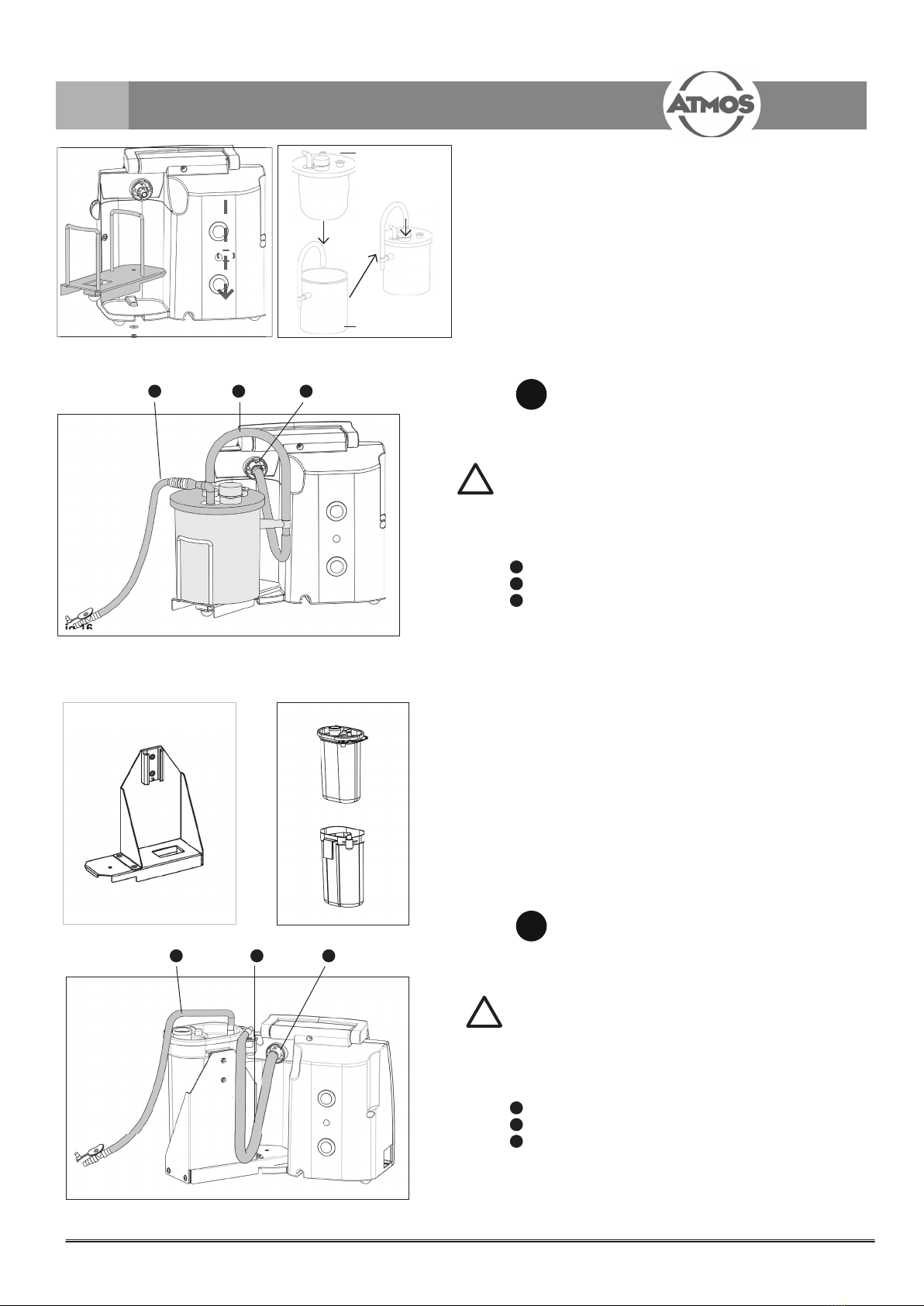
15 Vacuum channel
16 Vacuum hose
17 Connection for vacuum hose
7.3 Connect hose
Fig.16.
Insert the Medi-Vac®bag into the Medi-Vac®canister.
Close the canister tightly at all sides.
Check again for density, otherwise no
vacuum can be built up.
Insert the vacuum hose.
Only bags with integrated bacterial lter may be used!
!
7.4 Holder for Serres®secretion
canister
Plug the screw thread of the holder from the top into the
borehole at the bottom side of the device.
Screw it together with the provided screw nut.
7.5 Assembling the Serres®secretion
canister system
7.6 Connect hose
Insert the Serres®bag into the Serres®canister.
Close the canister tightly at all sides.
Check again for density, otherwise no vacuum
can be built up.
Insert the vacuum hose.
Only bags with integrated bacterial lter may be used!
!
15 Vacuum channel
16 Vacuum hose
17 Connection for vacuum hose
Fig.16.
i
15 16 17
15 16 17
i

12
8.0 Cleaning / Disinfection
The way the suction device is used determines its reliability
and safety. These hygiene measures are indispensable
for protecting the patient and the user and for maintaining
a safe and reliable suction device. When the suction
device is used on another patient or in case the device
has been oversucked a professional reprocessing by
the manufacturer, by a certi ed ATMOS partner or by a
specialist, who is authorised by ATMOS, in compliance
with Medical Devices Operator Ordinance, Medical
Devices Act and BV-Med rules is required (please see
chapter 9.2 reprocessing).
8.1 Basic information
●The following cleaning measures must only be
performed if the device was used. When the device
is only rarely used then a function check must be
conducted at least every three months. Only when
you adhere to these points the function of the device
can be guaranteed.
●We recommend you to document any maintenance
work and also any exchange of parts.
●Please always wear disposable gloves for any work
you perform.
●Prior to cleaning the device please remove and dispose
all disposable parts like filter, fingertip and catheter.
●Prior to cleaning please remove the mains cable from
the device.
●The described measures for cleaning and disinfection
do not replace valid rules for operating the device!
●Please observe the instructions for use prescribed
by the manufacturers of disinfectants. Especially
regarding concentration and regarding their
suitability for use.
●Attention: The lid parts and silicone hoses might get
dyed by some disinfectants; a fact which does not take
effect on the attributes of the materials.
●Basically all parts (canister, cover, overflow safety and
hose) which come into contact with suction material
must be cleaned, disinfected. Single-use parts like, for
example, filters, catheter, fingertip must be exchanged
as soon as the device is used in another patient.
Please see the different instructions for cleaning
(chapter 8.3).
●In case the suction device is used in one patient only
device and accessories should, for hygienic reasons,
be cleaned and disinfected. Please see the different
instructions for cleaning (chapter 8.3).
●Cleaning in an automatic cleaner and disinfecter is
also possible (hose connector, secretion canister and
canister lid).
Thermal disinfection is carried out at 93° C.
8.1.1 Bacterial fi lter
● Exchange the bacterial filter when changing the
patient. ATMOS recommends: Replace the bacterial
filter after 14 days, even if there is no patient change.
● Replace the bacterial filter with a new filter if it is
discoloured, contaminated or oversucked.
● Pay attention to storing a sufficient number of
replacement bacterial filters.
8.1.3 Fingertip
●Fingertip is not included in delivery.
●Prior to using the device in another patient the ngertip must
be exchanged.
●In case the device is used in one patient only we recommend
for hygienic reasons the exchange of the ngertip every day.
8.1.4 Secretion canister
●The secretion canister must always be disinfected with an
instrument disinfectant recommended on page 14 in case the
device is used in another patient. Prior to disinfection please
take care to empty the canister and to rinse it with clear water
in order to increase cleaning e ciency.
Please observe the instructions for use of the relevant
disinfectant solution!
●In case the device is used in one patient only we recommend
disinfection of the canister every day as described above.
●For hygienic reasons we recommend to empty the secretion
canister after every suction procedure and to rinse it with clear
water.
Information on how to remove the canister you will nd in
chapter 5.2 on page 9.
8.1.2 Suction hose, hose connector and
vacuum hose
●Suction hose and hose connector must always be disinfected
with an instrument disinfectant recommended on page 14 in
case the device is used in another patient. Prior to disinfection
or autoclaving the parts must be rinsed with clear water for
at least 10 seconds in order to increase cleaning e ciency.
Please observe the instructions for use of the relevant
disinfectant solution!
●In case the parts are used in one patient only we
recommend an exchange every 4 weeks.
●In addition we recommend to thoroughly rinse hose, hose
connector and vacuum hose with clear water and to disinfect
them at least once a day as described above.
!
!
!
8.1.5 Canister lid
●The canister lid must always be disinfected with an instrument
disinfectant recommended on page 14 in case the device is
used in another patient. Prior to disinfection please take care
to remove the bacterial lter from the lid and to disassemble
the lid into its single components (lid, oat ball and hose
connector). Prior to disinfection the parts must be rinsed with
clear water for at least 10 seconds in order to increase cleaning
e ciency. Afterwards the parts have to be disinfected.
Fig 17.

13
8.0 Cleaning / Disinfection
o The complete surface of the hose rewind, the trolley and the
device support must always be cleaned with a damp (not wet)
cloth and disinfected with a surface disinfectant stated on page
14 prior to use the device in another patient.
o In case the device is used in one patient only the device
surface should be cleaned if it is contaminated however at least
once every week with a damp (not wet) cloth and afterwards be
disinfected with a surface disinfectant stated on page 14.
8.2 Oversuction
When is a suction unit oversucked?
A suction device is oversucked if suction material penetrated into
the interior of the device.
How can one realise that the suction device is contaminated?
Generally a reduced suction capacity is a sign of possible oversuction.
The ATMOS®C 161 Battery has a condensate collector at the bottom
of the device. For visual inspection please remove the cap. The
device is oversucked if humidity or contamination is visible in the
condensate collector.
Measures
The device must be reprocessed by the manufacturer or a certi ed
ATMOS partner if the device is oversucked or if any reservations exist
regarding the hygiene condition.
A contaminated suction device bears a risk for the patient as well as
for the caregiver. Therefore, we recommend regular checking of the
condensate collector.
8.3 Cleaning instructions
for use in another patient
For use in one patient
contamination
after suction procedure
1x per day
1x per week
every 2 weeks
every 4 weeks
Exchange bacterial lter X X X
Rinsing the suction hose X X
Disinfecting the suction hose X X
Exchanging the suction hose X
Exchanging the ngertip X X
Emptying the secretion canister X X
Rinsing the secretion canister X X
Disinfecting the secretion canister X X
Rinsing the lid components X X
Disinfecting the lid components X X
Cleaning of the device surface X X X
Wipe disinfection of the
device surface X X X
Rinsing the vacuum hose X X
Disinfection of the vacuum hose X X
Disinfection of the hose connector X X
Please observe the instructions for use of the relevant
disinfectant.
Information on how to remove the secretion
canister lid you will nd in chapter 5.2 on
page 9.
●We recommend to thoroughly rinse the lid and its
components with clear water after each suction
procedure in case the device is used in one patient
only.
Attention! Please remove bacterial lter prior to
this!
8.1.6 Device surface
●Prior to use the device in another patient the
complete device surface must always be cleaned
with a damp (not wet) cloth and disinfected with a
surface disinfection solution stated on page 14.
●In case the device is used in one patient only
the device surface should be cleaned if it is
contaminated however at least once every week with
a damp (not wet) cloth and afterwards be disinfected
with a surface disinfectant stated on page 14.
Discolouration of the surface could occur by some
disinfectants; a fact which does not take e ect on
the attributes of the material.
Prior to cleaning please make sure to disconnect the
device from the mains!
The device may never be autoclaved, rinsed under
running water or immersed into any liquids!
8.1.7 Rinsing Canister
●The rinsing canister must always be disinfected in
case the device is used in another patient. Prior to
disinfection please take care to empty the canister
and to rinse it with clear water in order to increase
cleaning e ciency.
●In case the device is used in one patient only we
recommend disinfection of the canister every week.
●The rinsing canister may only be cleaned with a ph
neutral cleaning liquid which does not contain the
following ingredients: ammoniac, amines, amides,
phenol derivates, anionic tensides.
●The disinfection is exclusively allowed with a
disinfectant which does not contain the following
ingredients: alcohol, aromatic hydrocarbons,
ammonia, amine.
●Cleaning in a dishwasher is possible when using ph
neutral cleaning liquids (5 cycles)
8.1.8 Accessories
●Hose rewind (313.0007.0) /
Trolley (320. 0070.0) /
Device support (313.0012.0)
!
!
!
!
!

14
8.0 Cleaning
8.4 Recommended disinfectants for instruments
Disinfectant Contents (in 100 g) Manufacturer
GIGASEPT FF Succindialdehyde 11.0 g Schülke & Mayr, Norderstedt
(Application concentrate) Dimethoxytetrahydrofurane 3.0 g not for rinsing canister
Corrosion inhibitors
Non-ionic tensides and fragrances
Sekusept PLUS1Glucoprotamine 25.0 g Ecolab, Düsseldorf
(Application concentrate) Non-ionic tensides not for rinsing canister
Solvents, complexing agents
8.5 Recommended disinfectants for surfaces
Disinfectant Contents (in 100 g) Manufacturer
ATMOS®Green & Clean SK Alkyldimethylbenzylammoniumchloride <1 g Metasys, Rum (Austria)
(application solution) Dialkyldimehtylammoniumchloride <1 g
Alkyldimethylethylbenzylammoniumchloride <1 g
Dismozon pur Magnesium peroxyphthalate 80 g Bode Chemie, Hamburg
(granulate) Hexahydrate
End of product 12/2014
Dismozon plus Magnesium peroxyphthalate 95,8 g Bode Chemie, Hamburg
(granulate) Hexahydrate
Kohrsolin FF Glutaral 5 g Bode Chemie, Hamburg
(application solution) Benzyl-C12-C18-alkyldimethyl- 3 g not for rinsing canister
ammoniumchloride
Didecyldimethylammoniumchloride 3 g
Mikrozid sensitive wipes Quaternary Ammonium compounds 0.26 g Schülke & Mayr, Norderstedt
Perform Pentakalium bis(peroxymonosulphate)- 45.0 g Schülke & Mayr, Norderstedt
(application solution) bis(sulphate)
Bacillol 30 Foam Ethanol 14.0 g Bode Chemie, Hamburg
Propan-2-ol 10.0 g not for rinsing canister
Propan-1-ol 6.0 g
Alkylamino-
propylglycine < 1 g
Mikrobac forte Benzyl-C12-C18-alkyldimethyl- 19.9 g Bode Chemie, Hamburg
ammoniumchloride
N-(3-Aminopropyl)-N-dodecylpropan
1,3-diamin
Discolouration may result if disinfectants containing aldehydes and amines are used on the same object.

Cleaning and servicing plan for ATMOS®A / C 161 Battery
start date: name of the item: serial number:
* Before rst time operation of a brand new device, respectively a technically as well as hygienically proper device.
day
cleaning
secretion
containe-
hälter
cleaning
canister
lid
cleaning
of the
housing
exchange of
bacterial fi lter
exchange
of fi ngertip
exchange of
suction hose,
1.3 m
cleaning/exchange performed
name signature
exchange* exchange* exchange*
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchangeexchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
exchange
daily, respectively after each use
daily, respectively after each use
daily, respectively after each use
ATMOS MedizinTechnik GmbH & Co. KG
Ludwig-Kegel-Straße 16 / D-79853 Lenzkirch
Telefon: +49 (0)7653-689-0 / Fax: +49(0)7653-689-292
and other products must be avoided. The intervals stated in the list are
non-binding guide values. Depending on the use shorter intervals may be
necessary.
For each patient a new or technically as well as hygienically proper
suction device must be used. Otherwise there is high and acute danger
of infection for the patient, the user and any third person!
Special notes:
Before operating the suction device, the user has to make sure that the device
functions and is in good order and condition. The user has to observe the
instructions in the operating manual as well as all other safety-related and
maintenance information enclosed.
For cleaning and disinfection only agents which are recommended by the
manufacturer, may be used.
Only sterile, single-use suction catheters may be used for suctioning. They
have to be exchanged before each suction process. During use utmost
attention to hygiene (e.g. disinfection of hands, wearing single-use gloves) is
indispensable. After each use the secretion canister and the hose must be
rinsed thoroughly with water. During storage the contamination of the device

Important notes
General information
The way the suction device is used determines its reliability and safety.
These hygiene measures are indispensable for protecting the patient
and the user and for maintaining a safe and reliable suction device.
If there is a change of patient or ownership then the device must
be reprocessed acc. to Medical Devices Operator Ordinance,
Medical Devices Act and BVMed guidelines. An oversucked
(contaminated) device must be repaired by the manufacturer, by
a certifi ed ATMOS partner or an ATMOS authorized, specialized
dealer.
This cleaning and servicing plan as well as the relevant notes result
from many years of experience. Depending on the use and the user’s
experience shorter intervals may be necessary.
According to this cleaning and servicing plan the following consumables
have to be changed:
suction catheter, length: 50 cm
Disconnect the mains plug from the socket before commencing
with cleaning and disinfection!
Please observe the notes in the operating instructions, especially
regarding the recommended agents.
Cleaning of the secretion canister
Please empty the secretion canister after each use, rinse it thoroughly
with clear water and clean it with washing-up liquid. Tenacious
contaminations can be removed with a standard bottle brush.
Cleaning of the canister lid
The bacterial lter must be removed before cleaning, please use
single-use gloves. Please demount the canister lid after each use
and rinse it thoroughly. The lid must be absolutely dry before reuse.
Please pay attention to a correct function of the over ow safety when
mounting the lid.
Bacterial fi lter
The bacterial lter prevents penetration of micro organisms and
secretion into the device, respectively blowing out from it and is
therefore a protection for the user and the device. Replace the
bacterial lter with a new lter if it is discoloured, contaminated or
oversucked. Exchange the bacterial lter when changing the patient.
ATMOS recommends: Replace the bacterial lter after 14 days, even
if there is no patient change. In order to increase the service life of
bacterial lters, it is recommended to empty the secretion canister
when it is half-full. Always use the original ATMOS® bacterial lter
The suction device may not be operated without bacterial lter!
Hose connection/fi ngertip
The ngertip connects the suction hose to the suction catheter. As the
ngertip is in direct contact with secretion, and it is di cult to clean, we
recommend a daily exchanged. When the device is used for another
patient the ngertip must be exchanged immediately.
Suction Hose
The suction hose conducts the secretion from the suction catheter
to the canister. In order to prevent secretion from drying, the hose
must be thoroughly rinsed with clear water after each use. The water
can be sucked into the secretion canister. Please ll the secretion
canister only half. Frequent cleaning and disinfection may discolour
and embrittle the hose. Therefore, a monthly exchange of the suction
hose is recommended.
Cleaning of the device (housing)
When the device is contaminated but at least once per week the
housing must be wiped o with a moist (but not wet) cloth. A weekly
disinfection is recommended.
Never irrigate the device with water and never emerge it into any
liquid.
Cleaning/disinfection
To improve the cleaning e ect, standard washing-up liquid can be
added to the warm water. In the case of tenacious contamination the
parts should be steeped in water for a length of time or they may be
removed with a soft brush or cloth. After thorough cleaning, canister,
ngertip and hoses can be disinfected with a disinfection agent (see
operating instructions).
Cleaning in an automatic cleaner and disinfecter is also possible
(hose connector, secretion canister and canister lid).
Thermal disinfection is carried out at 93° C.
.
ATMOS MedizinTechnik GmbH & Co. KG
Ludwig-Kegel-Straße 16 / D-79853 Lenzkirch
Telefon: +49 (0)7653-689-0 / Fax: +49(0)7653-689-292
Never irrigate the device with water and never emerge it into any
To improve the cleaning e ect, standard washing-up liquid can be
added to the warm water. In the case of tenacious contamination the
parts should be steeped in water for a length of time or they may be
removed with a soft brush or cloth. After thorough cleaning, canister,
ngertip and hoses can be disinfected with a disinfection agent (see
Never irrigate the device with water and never emerge it into any
To improve the cleaning e ect, standard washing-up liquid can be
added to the warm water. In the case of tenacious contamination the
parts should be steeped in water for a length of time or they may be
removed with a soft brush or cloth. After thorough cleaning, canister,
ngertip and hoses can be disinfected with a disinfection agent (see
Never irrigate the device with water and never emerge it into any
Cleaning/disinfection
To improve the cleaning e ect, standard washing-up liquid can be
added to the warm water. In the case of tenacious contamination the
parts should be steeped in water for a length of time or they may be
removed with a soft brush or cloth. After thorough cleaning, canister,
ngertip and hoses can be disinfected with a disinfection agent (see
operating instructions).
Never irrigate the device with water and never emerge it into any
Cleaning/disinfection
To improve the cleaning e ect, standard washing-up liquid can be
added to the warm water. In the case of tenacious contamination the
parts should be steeped in water for a length of time or they may be
removed with a soft brush or cloth. After thorough cleaning, canister,
ngertip and hoses can be disinfected with a disinfection agent (see
operating instructions).
To improve the cleaning e ect, standard washing-up liquid can be
added to the warm water. In the case of tenacious contamination the
parts should be steeped in water for a length of time or they may be
Suction Hose
The suction hose conducts the secretion from the suction catheter
to the canister. In order to prevent secretion from drying, the hose
must be thoroughly rinsed with clear water after each use. The water
can be sucked into the secretion canister. Please ll the secretion
canister only half. Frequent cleaning and disinfection may discolour
and embrittle the hose. Therefore, a monthly exchange of the suction
When the device is contaminated but at least once per week the
Suction Hose
The suction hose conducts the secretion from the suction catheter
to the canister. In order to prevent secretion from drying, the hose
must be thoroughly rinsed with clear water after each use. The water
can be sucked into the secretion canister. Please ll the secretion
canister only half. Frequent cleaning and disinfection may discolour
and embrittle the hose. Therefore, a monthly exchange of the suction
When the device is contaminated but at least once per week the
Disconnect the mains plug from the socket before commencing
with cleaning and disinfection!
Please observe the notes in the operating instructions, especially
Please empty the secretion canister after each use, rinse it thoroughly
Cleaning in an automatic cleaner and disinfecter is also possible
(hose connector, secretion canister and canister lid).
Thermal disinfection is carried out at 93° C.
Disconnect the mains plug from the socket before commencing
with cleaning and disinfection!
Please observe the notes in the operating instructions, especially
Please empty the secretion canister after each use, rinse it thoroughly
Cleaning in an automatic cleaner and disinfecter is also possible
(hose connector, secretion canister and canister lid).
Thermal disinfection is carried out at 93° C.
suction catheter, length: 50 cm
suction catheter, length: 50 cm
suction catheter, length: 50 cmsuction catheter, length: 50 cm
Never irrigate the device with water and never emerge it into any
liquid.
Cleaning/disinfection
To improve the cleaning e ect, standard washing-up liquid can be
added to the warm water. In the case of tenacious contamination the
parts should be steeped in water for a length of time or they may be
removed with a soft brush or cloth. After thorough cleaning, canister,
ngertip and hoses can be disinfected with a disinfection agent (see
suction catheter, length: 50 cm
suction catheter, length: 50 cm
suction catheter, length: 50 cmsuction catheter, length: 50 cm
Never irrigate the device with water and never emerge it into any
liquid.
Cleaning/disinfection
To improve the cleaning e ect, standard washing-up liquid can be
added to the warm water. In the case of tenacious contamination the
parts should be steeped in water for a length of time or they may be
removed with a soft brush or cloth. After thorough cleaning, canister,
ngertip and hoses can be disinfected with a disinfection agent (see
white Ø 4 mm
green Ø 4.7 mm
orange Ø 5.3 mm
Bacterial lter
Fingertip
Suction hose
1.30 m
Float ball

17
9.1 Basic information
● Carry out a visual inspection of the unit prior to each
use including hoses, collection canister and connection
cable. Damaged cables and hoses must be replaced
immediately.
● Maintenance, repairs and period tests may only be
carried out by persons who have the appropriate technical
knowledge and are familiar with the product. To carry out
these measures the person must have the necessary test
devices and original spare parts.
ATMOS recommends: work should be carried out by
an authorized ATMOS-service partner. This ensures
that repairs and testing are carried out professionally,
original spare parts are used and warranty claims remain
una ected.
● Please comply with the country-speci c guidelines
regarding regular testing especially for the electrical
safety. ATMOS recommends a test every 24 months.
● For repair, this device can be returned to ATMOS.
● Before returning the device for repair, clean and
afterwards disinfect all secretion canister parts and hose
parts. The device´s surface also has to be disinfected.
● ATMOS cannot guarantee perfect functioning neither will
it be liable for damage to people or property if:
• Any non-original ATMOS parts are used,
• the user instructions given in this manual are not
followed exactly or are disregarded,
• assembly, resetting, alterations, extensions
and repairs are not carried out by people authorised
by ATMOS.
● No warranty rights shall exist in the event of damage or
failure caused by the use of non-ATMOS®accessories or
non-ATMOS®consumables.
● In order to protect the user, the ATMOS®A / C 161
Battery must be in good condition with regard to technics
and hygiene prior to passing on. If there is a change of
patient or ownership then the device must be reprocessed
acc. to Medical Devices Operator Ordinance, Medical
Devices Act and BVMed guidelines. An oversucked
(contaminated) device must be repaired by the
manufacturer, by a certi ed ATMOS partner or an ATMOS
authorized, specialized dealer.
● Pay attention to regulations and instructions valid for the
respective application range.
9.0 Maintenance and servicing
condensate
collector for a
quick view control
of a possible
contamination
Fig.
9.2 Reprocessing
When is a suction unit oversucked?
A suction device is oversucked if suction material penetrated
into the interior of the device.
How can one realise that the suction device is
contaminated?
Generally a reduced suction capacity is a sign of possible
oversuction.
The ATMOS®C 161 Battery has a condensate collector at
the bottom of the device ( g. 1). For visual inspection please
remove the cap. The device is oversucked if humidity or
contamination is visible in the condensate collector.
Measures
The device must be reprocessed by the manufacturer or a
certi ed ATMOS partner if the device is oversucked or if any
reservations exist regarding the hygiene condition.
A contaminated suction device bears a risk for the patient as
well as for the caregiver. Therefore, we recommend regular
checking of the condensate collector.
The way the suction device is used determines its
reliability and safety. These hygiene measures described
in the last chapter are indispensable for protecting the
patient and the user and for maintaining a safe and
reliable suction device.
When you can ensure the device was not oversucked
then perform a reprocessing acc. to Medical Devices
Operator Ordinance, Medical Devices Act and BVMed
guidelines. The reprocessing consists of cleaning,
surface disinfection as well as the exchange of
consumables. An ATMOS set of consumables is
available for this purpose.
If you cannot exclude that the device was
oversucked then the device must be repaired by the
manufacturer, by a certifi ed ATMOS partner or an
ATMOS authorized, specialized dealer. Subsequently
the device may be operated again.
9.3 Battery handling
● Prior to rst use, the battery must be fully charged!
●Total discharge may destroy the battery. Therefore, please
fully recharge the batteries of the ATMOS®A / C 161
Battery every 3 months, even if the device is not used.
●Battery-run devices should only be stored when they are fully
charged.
●If the device was not in operation for a long period of time,
the full capacity of the battery can only be achieved when
4 complete recharging and discharging cycles have been
completed.
●Used batteries should be replaced immediately by the
customer service. The mains operation of the device
with used batteries can destroy the charging electronics
respectively excessive power consumption of the device
may result in a spontaneous cut-o .
●Heat destroys the batteries. Therefore, please prevent
them from direct solar radiation and keep them away from
radiators. The perfect storage temperature is between
8 – 15° C.
●The battery should be exchanged by the service
department if the available capacity (operating time) is
less than 80 % compared with a new battery.
●Batteries are run-down after approx. 800-900 charging
cycles.
●Correct handling of the rechargeable batteries prolongs
their maximum service life.
●Rechargeable batteries are wearing parts and therefore
excluded from the 2 years‘ warranty!
1

18
9.0 Maintenance and servicing
9.4 Sending in the device
1. Remove and properly dispose of consumables.
2. Clean and disinfect the products and accessories according to the operating instructions.
3. Place used accessories with the device.
4. Fill in the form QD 434 „Delivery complaint / return shipment“ and the respective Decontamination certi cate.
• This form is enclosed to each delivery and can be found at www.atmosmed.com.
5. The device must be well padded and packed in suitable packaging.
6. Place the form QD 434 „Delivery complaint / return shipment“ and the respective decontamination certi cate in an
envelope.
7. A x the envelope to the outside of the package.
8. Send the product to ATMOS or to your dealer.

19
10.0 Troubleshooting
Prior to dispatch, the ATMOS®A / C 161 Battery was subjected to an extensive functional test. If, nevertheless, a failure
should appear, you may possibly clear it yourself if you follow these notes:
Problem Possible causes Remedy
• Unit does not start - Discharged battery - Connect the battery charging
power pack to the device. The
battery should be recharged for
1 – 2 hours prior to operation with
battery.
- Loose power plug of the charging
device
- Check all plug and socket connec-
tions. Pay attention to the control
lamp, it must be illuminated when
all connections are o.k.
• Insucient perfor-
mance
- Discharged battery - Recharge the battery.
- Filter is blocked - Exchange the lter.
• 1. Low or no vacuum
is indicated
- 1.1 DDS bacterial lter is missing - Insert DDS bacterial lter
- 1.2 Leakages within the hose system
or in collection canister lid
- Check collection canister lid and
hose system on tight tting.
- Connect the lter once again to the
connection nozzle.
- Check the suction lid on tight and
correct tting.
- 1.3 Secretion or blood has been su-
cked in and valve plates of the pump
are contaminated
- Unit has to be returned for repair.
• 2. High vacuum is
indicated
- 2.1 DDS bacterial lter is blocked. - Exchange DDS bacterial lter
- 2.2 Float of overow safety closes
the collection canister inlet
- Check collection canister inlet;
if necessary empty secretion
canister, clean the oversuction
protection and check oat ball for
exibility.
• Device switches o
automatically when
switched on
- Applied vacuum (over 40%) - Venting the vacuum, then recom-
missioning is possible

20
11.0 Accessories, consumables and spare parts
DDS bacterial fi lter /
oversuction stop
Hose connection,
(fi ngertip)
Suction catheter
Plak-VacTM toothbrush
Trolley on
4 castors
Cover for
secretion canister
(incl. spare DDS fi lter)
Grad.
secretion canister 1 l
Rewind system
for suction hoses
Separate
rinsing canister set
Basket for catheters
Device support
REF
11.1 Accessories for ATMOS®A / C 161 Battery
Grad. secretion canister, 1 l DDS, blue, PSU 313.0015.0
Grad. secretion canister, 1 l DDS, transparent, PSU 313.0017.0
Secretion canister lid, 1 l DDS, blue, with spare bacterial filter 313.0006.0
Separate rinsing canister set for ATMOS®A- and C- class
incl. lid and support
313.0008.0
Hose connector for hoses Ø 6 mm 000.0836.0
Hose rewind for suction hoses A- and C- class 313.0007.0
Charging device for ATMOS®Battery, 100 - 240 V~, 50/ 60 Hz 011.1334.0
2 pole mains cable 008.0920.0
Carrying bag, black 313.0011.0
Car power cable for ATMOS®C 161 Battery,
connection: 12 V-
313.0436.0
Device support for ATMOS®A- and C- class 313.0012.0
Trolley on 4 castors, for A- and C- class 320.0070.2
Support for Receptal®external canister 1 l and 1.5 l 313.0009.0
Support for Medi-Vac®external canister 1 l 313.0010.0
Support for Serres®external canister 1 l 313.0413.0
11.2 Consumables
Bacterial filter for DDS secretion canister, 10 pcs. 340.0054.0
Set of consumables for ATMOS®A- and C-class DDS 313.0160.0
General accessories
Receptal®external canister 1.5 l 310.0221.0
Serres®external canister 1 l 312.0465.0
Medi-Vac®canister 1 l 312.0473.0
Basket for catheters, L = 340 mm 444.0140.0
Basket with standard rail holder 320.0075.0
Suction hoses / fingertip
Plak-VacTM tooth brush with suction mechanism 000.0821.0
Suction hose, silicone, Ø 6mm, minimum purchase 5 m 006.0009.0
Suction hose, silicone, Ø 6 mm, L = 1.30 m, 1 pc. 000.0013.0
Suction hose, disposable, Ø 6 mm, L = 1.30 m, 10 pcs. 006.0057.0
Suction hose, disposable, Ø 6 mm, L = 2.10 m, 50 pcs. 006.0059.0
Fingertip, sterile, not autoclavable, 10 pc. 000.0347.0
Suction bags
Receptal®suction bag 1.5 l, not autoclavable, 50 pcs. 310.0222.2
Medi-Vac®suction bag 1 l, not autoclavable, 50 pcs. 312.0474.0
Serres®suction bag, 1 l, not autoclavable, 36 pcs. 312.0466.0
Suction catheters
Suction catheter, size: CH 12, L = 50 cm, 100 pcs.
Ø 4 mm, white, straight, central opening, 2 small lateral openings, suction connec-
tion Ø 6 mm, sterile, not autoclavable
000.0294.0
Suction catheter, size: CH 14, L = 50 cm, 100 pcs.
Ø 4.7 mm, green, straight, central opening, 2 small lateral openings, suction con-
nection Ø 6 mm, sterile, not autoclavable
000.0295.0
Suction catheter, size: CH 16, L = 50 cm, 100 pcs.
Ø 5.3 mm, green, straight, central opening, 2 small lateral openings, suction con-
nection Ø 6 mm, sterile, not autoclavable
000.0296.0
Grad.Grad.
This manual suits for next models
6
Table of contents
Other Atmos Medical Equipment manuals

Atmos
Atmos S 351 NATAL User manual

Atmos
Atmos E 2 User manual

Atmos
Atmos C 361 User manual

Atmos
Atmos Variotherm plus User manual
Atmos
Atmos S 042 NPWT User manual

Atmos
Atmos Twin Record 55 User manual

Atmos
Atmos A 161 Reference manual
Atmos
Atmos LC 16 User manual

Atmos
Atmos LC 27 User manual

Atmos
Atmos iQam User manual

Atmos
Atmos Strobo 21 LED User manual

Atmos
Atmos Variotherm plus User manual

Atmos
Atmos Record 55 User manual

Atmos
Atmos C 451 User manual
Atmos
Atmos S 61 CORIAN integral User manual

Atmos
Atmos C 161 User manual

Atmos
Atmos C 161 User manual
Atmos
Atmos C 051 Thorax User manual

Atmos
Atmos i View COLPO User manual

Atmos
Atmos Record 55 User manual