Biolase Waterlase User manual

Waterlase Fractional Handpiece
Instructions for Use
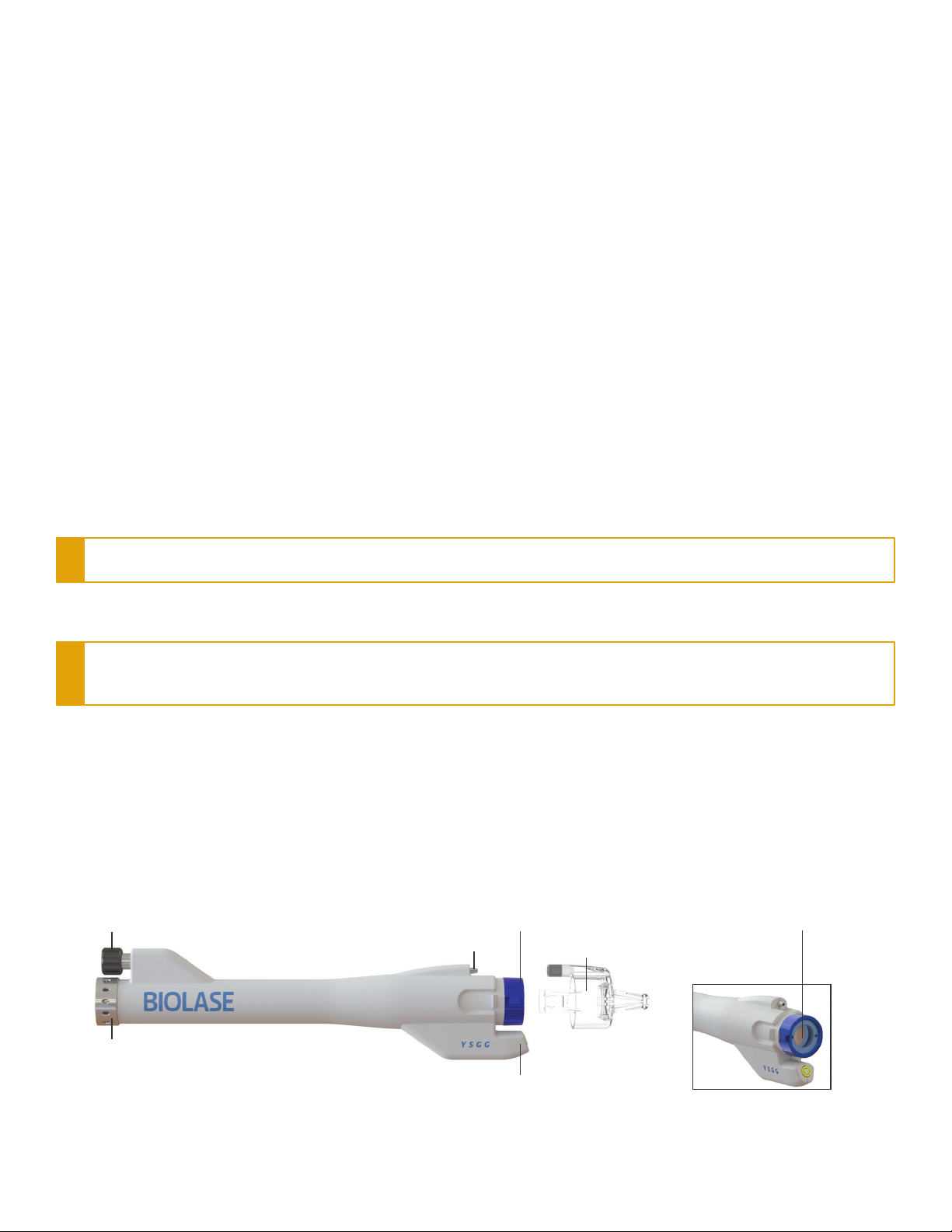
Introduction
The Waterlase Fractional Handpiece™is designed to work exclusively with a Fractional-ready Waterlase iPlus All-Tissue Laser. It utilizes Waterlase advanced laser
technology to atomize water and ablate skin and/or reshape soft tissue with minimal trauma and leverages the absorption properties of the Er,Cr:YSGG 2780nm
laser wavelength in skin, generating 10 microbeams in a single line per laser pulse. Treatment is always performed in contact mode; the Disposable Applicator
(attached to the distal end of the Fractional Handpiece) is in contact with the skin when the laser is activated.
Section 1: Indications for Use
This Fractional Handpiece is indicated for use in dermatology for skin resurfacing.
Section 2: Contraindications, Warnings, and Precautions
Contraindications, Warnings, and Precautions related to the use of the Waterlase iPlus laser are provided in the Waterlase iPlus User Manual. Clinician must know
and adhere to state guidelines and regulations when purchasing and using this accessory. Check with your state and/or local government for required permits or
other applicable laws. The Fractional Handpiece is for skin resurfacing, this is an extra-oral Handpiece and is not intended for intra-oral use.
Only licensed professionals who have reviewed and understood this Instruction for Use should use this device. Practioners must use their own clinical judgment and
professionalism in determining all aspects of treatment, technique, proper power settings, interval, duration, etc. BIOLASE assumes no responsibility for parameters,
techniques, methods, or results.
• Do not use the Fractional Handpiece on patients who are pregnant
• Do not use the Fractional Handpiece on patients undergoing treatment for skin cancer
The laser emits both visible and invisible laser radiation which could cause eye damage if proper safety measures are not taken. Doctor, patient, assistant, and all
others inside or entering the operatory must always wear appropriate laser protection eyewear for the laser wavelength (2780nm, OD4) whenever the laser is in use.
Patient-specific goggles (Biolase p/n 2201293) are included and must be worn by the patient at all times during treatment.
CAUTION: Prior to use, inspect eyewear for pitting and cracking; do not use if damaged. Always check the eyewear specifications etched on the glasses to
ensure they offer the protection required for the laser wavelength.
!
• Do not look directly into the beam or at specular reflections while the laser is operational
• Do not point the laser beam towards reflective surfaces, including jewelry or protective goggles
CAUTION: Laser plume may contain viable tissue particulates; special care must be taken to prevent infection from the laser plume generated by vaporization
of virally or bacterially infected tissue when using minimal water spray. Ensure that all appropriate protective equipment, including filtration masks, are used
at all times during procedures performed with this laser device.
!
Use caution when treating patients who have the following conditions:
• History of keloids or hypertropic scarring
• History of herpes
• Isotretinoin (Accutaine/Raccutane) use
• History of vitiligo
AUXILLARY AIR INPUT AUXILIARY AIR OUTPUT
(PLUME REMOVAL)
SPRAY MIXER (AIR/WATER)
LASER OPTICS HOUSING
DISPOSABLE APPLICATOR
COLLAR
OPTICAL LENS
Figure 1

Section 3: Overview
Fractional Handpiece and Disposable Applicator
Specications
Maximum
power Setting:
Up to 9 W at 15 Hz Spot spacing: 0.9 mm & 1.0mm* in Y direction (*based on movement speed of 1.5 cm/second)
Total energy/pulse: 100 - 400 mJ Fluence per spot: 20 - 82 J/cm2
Energy per spot: 10 - 40 mJ Surface coverage 5% (single pass; *based on movement speed of 1.5 cm/second)
Spot Diameter: 250 μm
The Fractional Handpiece Assembly includes:
• (1) Fractional Handpiece
• (5) Single-use disposable applicators
• (1) Auxiliary air hose assembly with fastener clips
• (1) Handpiece holder attachment
• (1) Patient safety goggles
• Instructions for Use
Auxiliary Air Hose Assembly
LUER CONNECTOR TO
AUXILIARY AIR INPUT
ON HANDPIECE
CONNECTOR TO AUXILIARY AIR
OUTPUT ON LASER
FASTENER CLIPS
Figure 2

HANDPIECE HOLDER
The Handpiece Holder Attachment is designed to fit over the built-in Waterlase iPlus Handpiece
Holder on the laser console and, once in place, will support the Fractional handpiece as well as all
other Waterlase iPlus handpieces.
ASlide the attachment from the back toward the front onto the existing Handpiece Holder on the
laser console.
BClip the back under the edge of the existing holder.
CLower over the existing holder and snap into place.
Figure 3A
Figure 3
Figure 3B
Figure 3C
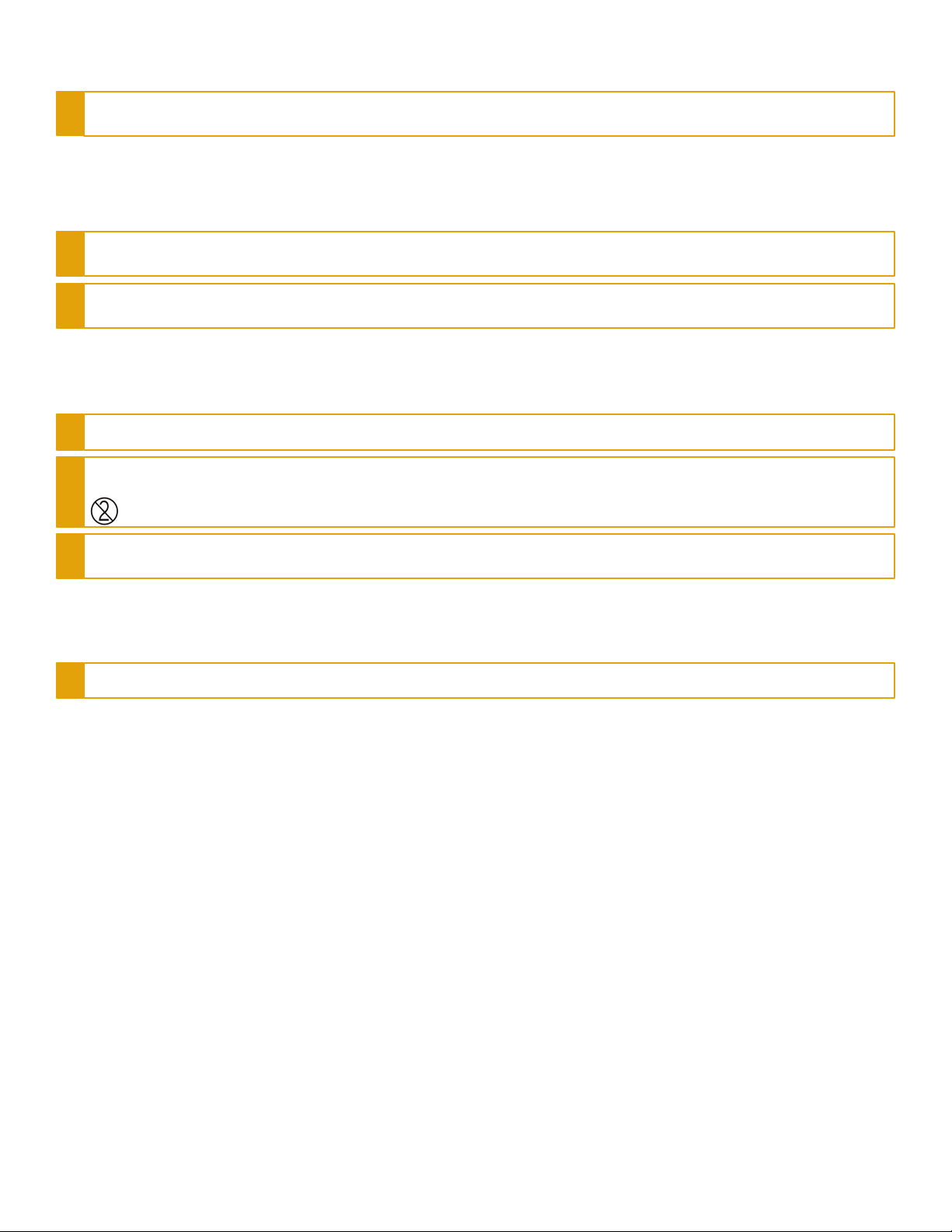
Section 4: Cleaning and Disinfection
CAUTION: The Waterlase Fractional Handpiece is non-sterile when shipped from the factory. DO NOT AUTOCLAVE. Doing so will damage the internal
optics. However, the Handpiece must be cleaned and disinfected prior to initial use and between patients to avoid cross-contamination.
!
The Handpiece
To clean and disinfect the Waterlase Fractional Handpiece, wipe the entire outer surface up to the laser optics housing using a cotton gauze and an appropriate
disinfecting solution, such as Cavicide™, or a similar quaternary ammonium compound product (containing 20% alcohol or less), and follow the manufacturer’s
instructions. Avoid getting any liquid or debris on the optical lens inside the laser optics housing.
CAUTION: Always inspect the optical lens for dirt and debris between uses; if any debris is visible on the lens, clean by gently wiping with a cotton swab
dampened with isopropyl alcohol. The isopropyl alcohol must be allowed to evaporate before the laser equipment is used.
!
CAUTION: Check the Handpiece for damage or wear prior to each use. It should be free of nicks, distortion, corrosion, or other signs of mechanical
degradation. If damage or wear is observed, discard the Handpiece as required by local recycle/waste disposal regulations.
!
THE DISPOSABLE APPLICATOR
As with the Fractional Handpiece, the Applicator is sold non-sterile and must be wiped with an appropriate disinfecting solution, such as Cavicide, or a similar
quaternary ammonium compound product (containing 20% alcohol or less), and follow the manufacturer’s instructions, prior to assembly.
CAUTION: DO NOT AUTOCLAVE, as the autoclave process may damage the Applicator and make it unusable.
!
CAUTION: The disposable Applicator is intended for single-use only to avoid cross-contamination between patients and to ensure the Handpiece performs
as indicated; re-using the Applicator will result in a less effective treatment outcome.
DO NOT REUSE. After one-time use, detach it from the Fractional Handpiece and discard in a medical waste sharps container.
!
CAUTION: High temperatures produced in the normal use of the Waterlase iPlus laser system may ignite some materials (e.g., cotton wool when saturated
with oxygen); flammable solutions or solvents used for cleaning and disinfecting must be allowed to evaporate before the laser equipment is used.
!
THE FIBER & AUXILIARY AIR HOSE
Always disinfect the Fiber Optic Cable and Auxiliary Air hose between patients by wiping them completely with an appropriate disinfecting solution such as Cavicide
or a similar quaternary ammonium compound product (containing 20% alcohol or less) and follow the manufacturer’s instructions.
CAUTION: DO NOT AUTOCLAVE. Autoclaving will damage the Fiber and air hose and make them inoperable.
!
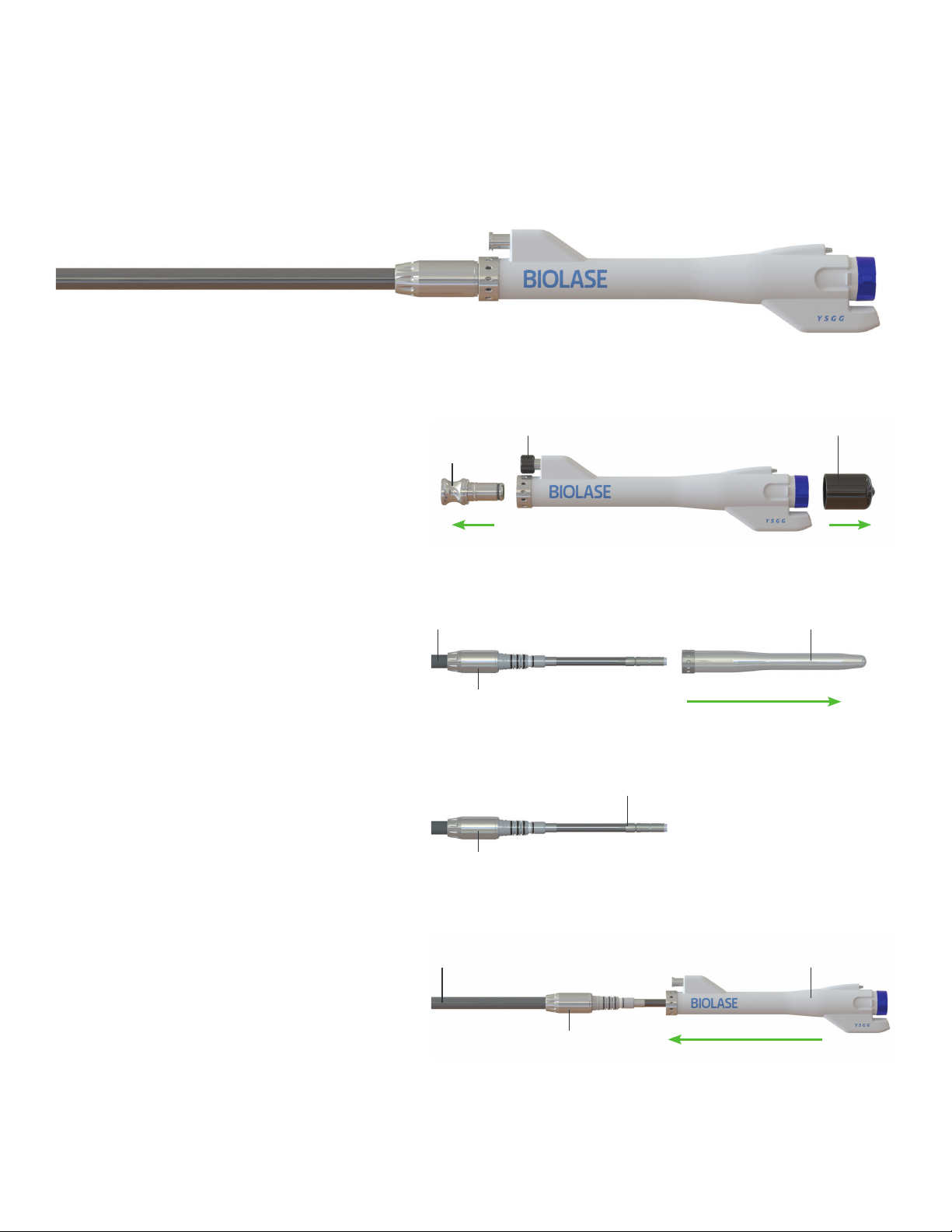
Section 5: Set-Up
Refer to the Waterlase iPlus User Manual for detailed instructions on system set-up, installation and operation.
CONNECTING THE FRACTIONAL HANDPIECE TO THE FIBER OPTIC CABLE
• If switching from a Gold or Turbo Handpiece to the Fractional Handpiece, always purge the Handpiece before disconnecting it from the Fiber Optic Cable.
• Make sure the system is in STANDBY mode, not in READY mode.
Figure 4C
FIBER SHAFT
CONNECTOR
Figure 4B
FIBER OPTIC CABLE PROTECTIVE COVER
CONNECTOR
Figure 4
A. Gently, but firmly, pull the laser optics protective cap and rear
plug from the Fractional Handpiece; be sure to save these, as
they will always be required when preparing the Handpiece for
cleaning and disinfection, and storage.
B. Hold the Fiber Optic Cable by the connector; remove the fiber
protective cover from the fiber shaft by firmly pulling it. Do not
apply excessive force. Be sure to save the cover.
C. Check the fiber shaft for any moisture and wipe with a dry, lint-
free tissue if any is present.
D. Carefully insert the Fractional Handpiece onto the fiber shaft
until it sits firmly against the connector and clicks into place.
(To disconnect the Fractional Handpiece, hold the Fiber Optic
Cable by the connector and firmly pull on the Handpiece until it
comes completely off the shaft. Do not apply excessive force.)
Figure 4A
REAR CAP
LASER OPTICS CAP
CONNECTOR
FIBER OPTIC CABLE HANDPIECE
Figure 4D
AUXILLARY AIR INPUT CAP
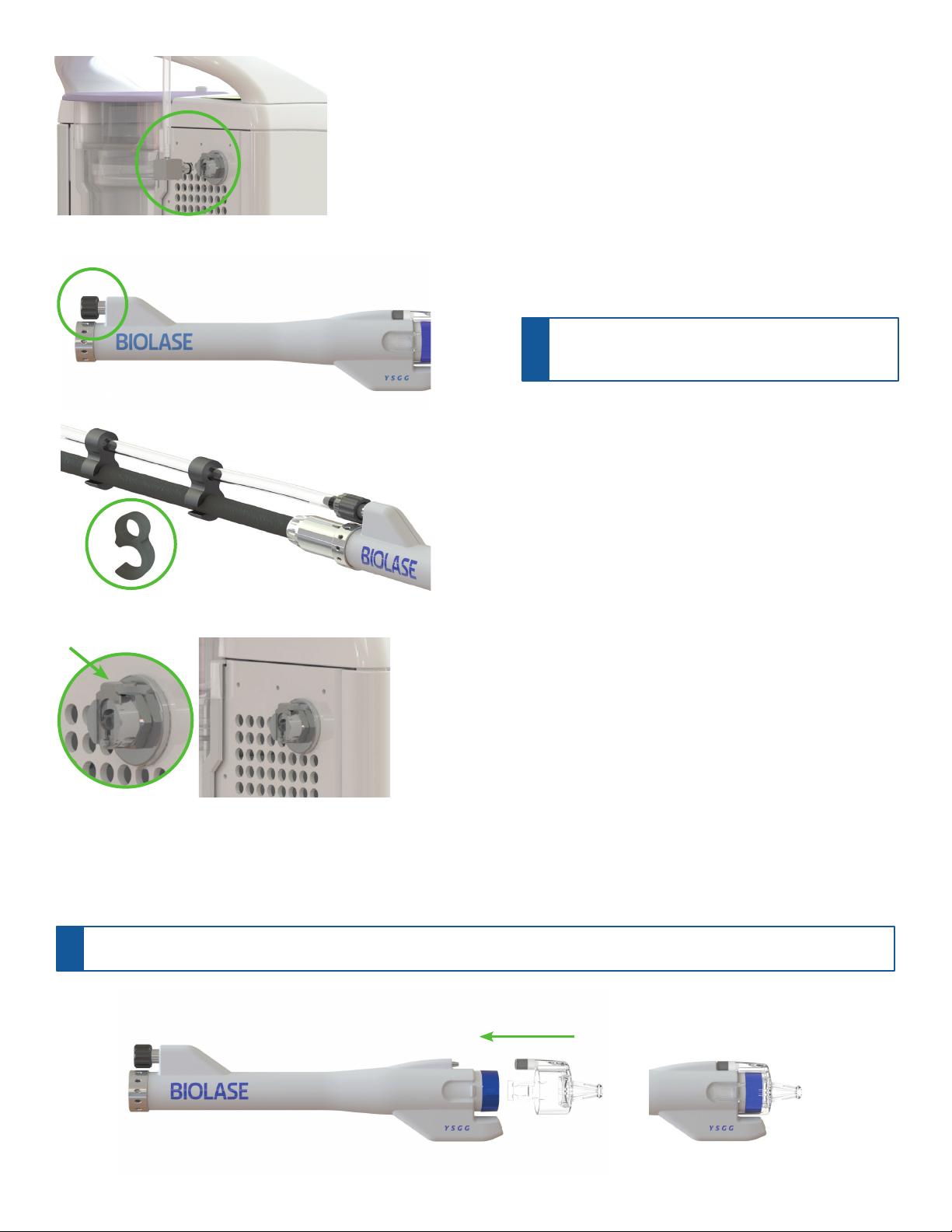
INSTALLING THE AUXILIARY AIR OUTPUT LINE ON THE
WATERLASE IPLUS
A. Insert the metal connector attached to one end of the auxiliary air hose
into the auxiliary air output port on the back of the iPlus laser.
B. Unscrew the auxiliary air input protective cap from the auxiliary air
input port and save; attach the end of the auxiliary air hose with the
black luer connector to the auxiliary air input port.
C. For ease of use, attach the clips threaded on the auxiliary air hose to
the Fiber Optic Cable.
D. To unplug the auxiliary air hose from the Waterlase iPlus laser, press
down on the release tab at the top of the of the auxiliary air output port
and gently pull on the connector to detach.
NOTE: The handpiece optics may become permanently
damaged if the auxiliary air output is not connected when
performing procedures using the Fractional Handpiece.
!
Figure 5A
Figure 5D
Figure 5C
Figure 6
ATTACHING THE DISPOSABLE APPLICATOR TO THE FRACTIONAL HANDPIECE
Attach the single-use disposable applicator to the Fractional Handpiece by pushing it onto the distal end of the Handpiece until it snaps into place. To remove the
applicator, lift either tab and pull; the tab will break off.
NOTE: Do not use a disposable applicator if it has been dropped on a hard surface or is cracked or damaged in any way. Sharp edges may cause injury to
the patient.
!
Figure 5B
Section 6: Operation
Refer to the Waterlase iPlus User Manual for instructions on how to turn on and activate the Waterlase iPlus All-Tissue Laser.
Make sure the Handpiece is properly connected to the Fiber Optic Cable and the auxiliary air hose is connected to the Waterlase iPlus.
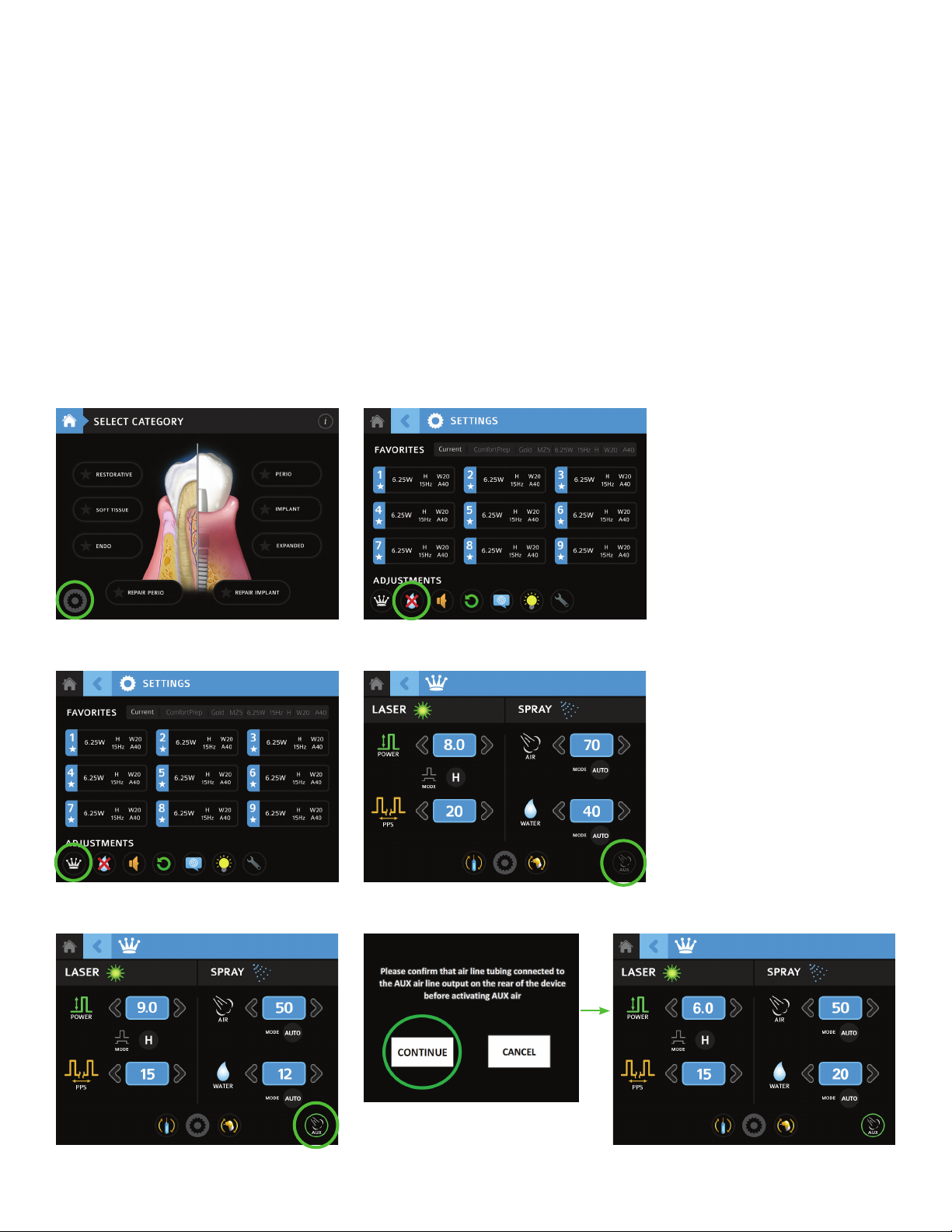
A. From the Home Menu, select Settings. (Figure 7A)
B. Press the Purge/Prime icon on the Settings screen, then select Prime. Press and hold Prime until the Fiber Optic Cable and Handpiece are primed with water;
water will spray from the end of the Handpiece. (Figure 7B)
C. Once the Prime function has completed, press the back arrow and then proceed to the Advanced screen by pressing the Advanced icon (crown). (Figure 7C)
D. The Auxiliary Air icon on the bottom right of the screen turns the Fractional function on and off. When it is off, the icon will appear dark. (Figure 7D)
E. Press the Auxiliary Air icon to initiate the Fractional function. (Figure 7E)
F. A screen will appear asking the user to confirm that the Auxiliary Air Output tubing is connected to the Auxiliary Air Output connector; if connected, press
“Continue;” the icon will light up. If “Cancel” is selected, the system will not initiate the Fractional function (the icon stays dark). (Figure 7F)
G. Default settings for Fractional will automatically appear on the screen. Place the system in READY mode to activate the Fractional air flow and begin lasing.
(Figure 7G)
To turn off the Fractional function, press the Auxiliary Air icon again; the icon will go dark.
Figure 7A Figure 7B
Figure 7C Figure 7D
Figure 7E
Figure 7F
Figure 7G
Section 7: Clinical Applications
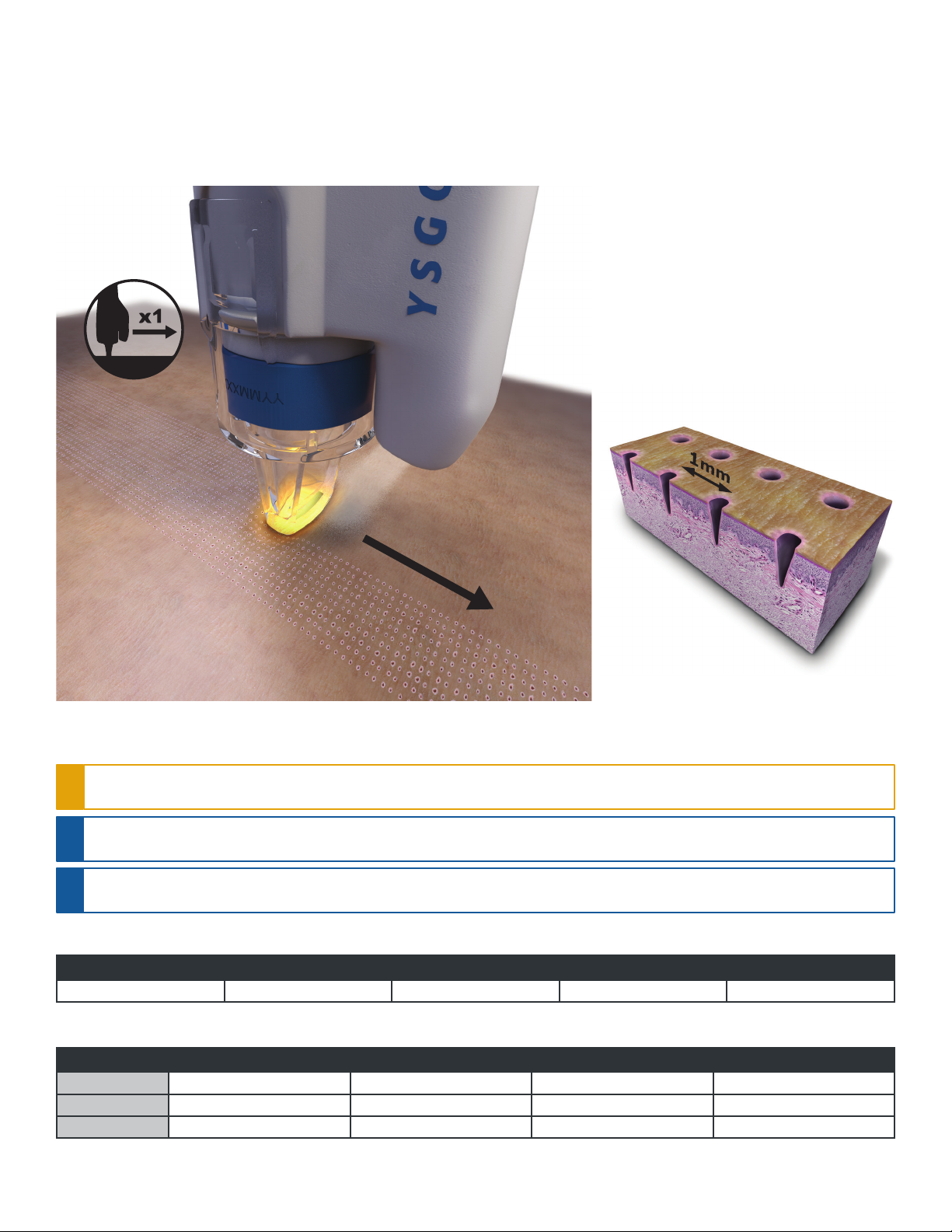
The Waterlase Fractional Handpiece generates 10 microbeams in a single line per laser pulse. Treatment is always in contact mode, i.e., the Applicator attached to
the distal end of the Fractional Handpiece is applied directly to the skin while the laser is activated.
A uniform, one-dimensional pattern of micro-holes is generated on the tissue surface. For maximum efficacy, the Handpiece needs to be constantly moving across
the skin surface at approximately 1.5cm/sec.
1
.
5
c
m
/
s
Figure 8
CAUTION: To avoid damaging the skin, DO NOT keep the Handpiece in a fixed position. Always keep the Handpiece constantly moving across the skin
surface. DO NOT make more than one pass per treatment session
!
NOTE: For optimum results, place the Applicator in contact with the treatment area and begin moving the Handpiece before firing the laser.
!
NOTE: If the Applicator has not been properly installed, the pattern of micro-holes will not be uniform or will be less than 10. Remove the Applicator and
replace it with a new one.
!
When the Fractional function is activated, the system will default to the following settings:
Power Frequency Air Water Mode
6W 15Hz 50% 20% H
However, some parameters may be adjusted by the user, within set boundaries:
Setting Min. Max. Options Mode
Power 2W 9W — H or S
Water 1% 20% ON/OFF/AUTO H or S
Air 1% 50% ON/OFF/AUTO H or S

It is also possible to switch from H mode (narrow coagulation zone) to S mode (shallow penetration in tissue), as needed, based on the patient’s skin type.
NOTE: Water, when combined with air, creates a spray that cools the treatment site and facilitates the effects of the laser energy applied.
!
Frequency cannot be modified, and remains fixed.
Refer to the Fitzpatrick Skin Type Scale before performing Fractional procedures to determine the best settings for use on a patient. For example, darker skin (Type
IV and above) may necessitate a higher power setting for the treatment to be effective, as well as a wider spot density (i.e., moving the handpiece faster than
1.5 cm/s), for preventing post-inflammatory hyperpigmentation (PIH).
Power settings should also be adjusted to compensate for the thickness of thinness of the tissue being treated. Periorbital tissue will require a lower setting, while the
forhead and cheek area require a higher setting.
CAUTION: Always monitor patient response; adjust power/water/air settings as needed for patient comfort.
!
Fitzpatrick Skin Type Scale
Type I Highly sensitive, always burns, never tans (pale white skin)
Type II Very sun-sensitive, burns easily, tans minimally (white skin).
Type III Sun-sensitive skin, burns moderately, tans uniformly (light brown skin)
Type IV Minimally sun-sensitive, burns minimally, always tans well (moderate brown skin)
Type V Sun-insensitive skin, rarely burns, tans profusely (dark brown skin)
Type VI Sun-insensitive, never burns (deeply pigmented dark brown to black skin)
• Always use a test spot prior to the initial treatment to assess the suitability of the selected settings and effect of the Fractional procedure for the individual patient.
• Adjust power and water settings, as needed, based on the patient’s skin type and condition, as well as the desired outcome of the procedure.
• Treatment normally causes minimal pain or discomfort to the patient. However, if the patient does experience pain or discomfort, stop treatment, and/or decrease
the power, increase the air setting or the water setting, or both; ice or a topical anesthetic may be applied prior to treatment if necessary.
For optimum results:
• Prior to treatment, make sure the skin has been thoroughly cleansed of all residual oil and/or dirt.
• Immediately post-treatment, wash the treatment area with water and apply a moisturizing cream or gel.
• Instruct the patient to apply moisterizier to the treatment area as often as necessary throughout the day for at least 2 weeks to keep the skin from drying out.
• The patient should avoid sun exposure as much as possible for 1 to 2 weeks after treatment and use a high SPF (>35) sunscreen for at least 3 weeks.
• No makeup should be worn for at least 1 week post-treatment to minimize inflammation and the risk of infection; the treatment area should be kept clean at all
times.
What to Expect Post-Treatment
• Short-term erythema (redness)
• Short-term edema (swelling)
• Short-term itching
• Scabbing
Adverse Eects
If the power level used is too high, or the air and water levels are not optimized for the patient’s skin type, the following may occur:
• Hyperpigmentation
• Hypopigmentation
• Blistering
• Erosion
Always monitor the patient for any sign of irritation or infection; if either occurs, promptly treat the condition with the appropriate medications to avoid delayed
healing, scarring, or the spead of infection.

Section 8: Warranty and Liability
Limited Warranty
For warranty information, refer to separate equipment warranty.
Limited Liability
BIOLASE, Inc. will not be liable for incidental, consequential, indirect or special damages of any kind including, but not limited to, damages for loss of revenue, loss of business
or business opportunity or other similar financial loss arising out of or in connection with the performance, use or interrupted use of the BIOLASE laser system(s) or any
BIOLASE materials.
Section 9: Reordering Accessories
To reorder system accessories, contact your authorized BIOLASE representative.
Part No. Description
6201818 Single-Use Disposible Applicators
6201819 Auxiliary Air Hose Assembly
4201667 Handpiece Holder Attachment
2201293 Patient Safety Goggles
7220002 Waterlase Fractional Handpiece

BIOLASE, Inc.
27042 Towne Centre Drive, Suite 270
Foothill Ranch, CA 92610 USA
Tele: +1 9 49.361.1200
Toll Free: 833.BIOLASE
Service: 800.321.6717
Fax: +1 949.273.6687 biolase.com
EUROPEAN REPRESENTATIVE
M T Promedt Consulting GmbH
Altenhofstrasse 80
D-66386 St. Ingbert/Germany
+49 6894 581020
www.mt-procons.com
P/N: 5201615 Rev F
Rx Only
Table of contents
Other Biolase Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual

















