Boehringer 3930 User manual

User Manual
Boehringer Laboratories, LLC
300 Thoms Dr.
Phoenixville, PA 19460
800-642-4945
Model 3930
Vacuum Assisted Venous
Drainage Controller w/
WAGD Suction Flowmeter
3930.003 Rev C P/N 34031 1 of 9
Released July 2014 User Manual VAVD Controller

WELCOME
Congratulations on your purchase of the Boehringer VAVD Regulator. We consider our regulators
to be the best in the world. We are confident it will provide you with reliable, trouble-free, safe
patient care and low cost of operation. This product is intended for use by clinicians properly
trained in the use of vacuum assisted venous drainage for cardiopulmonary bypass. The product is
intended for use on the order of a physician. Please read these instructions carefully.
Definition of Terms...................................................................................................................3
Indications ................................................................................................................................3
Contraindications......................................................................................................................4
Safety Information....................................................................................................................4
Operation/Features ..................................................................................................................5
Theory of Operation .................................................................................................................6
Installation ................................................................................................................................6
Pre-Use Check.........................................................................................................................7
Mode Selection.........................................................................................................................7
Clinical Use...............................................................................................................................7
Maintenance.............................................................................................................................8
Cleaning & Disinfection ............................................................................................................8
Troubleshooting........................................................................................................................8
Specifications ...........................................................................................................................9
Warranty and Repair ................................................................................................................9
Contents
3930.003 Rev C P/N 34031 2 of 9
Released July 2014 User Manual VAVD Controller

Parts .........................................................................................................................................13
VACUUM Air or other gases at a sub atmospheric pressure typically expressed as mmHg.
SUCTION A use of vacuum that causes a fluid or solid to be drawn into an interior space or to adhere to a
surface because of the difference between the external and internal pressures.
Alerts the user to the presence important operating and maintenance instructions in the
literature accompanying the device.
WARNING Alerts user to actions or conditions that could result in injury to user or patient.
CAUTION Alerts user to actions or conditions that can cause damage to the device or may result in
substandard performance of the device or system.
IMPORTANT Indicates an action that is emphasized to ensure proper operation of equipment.
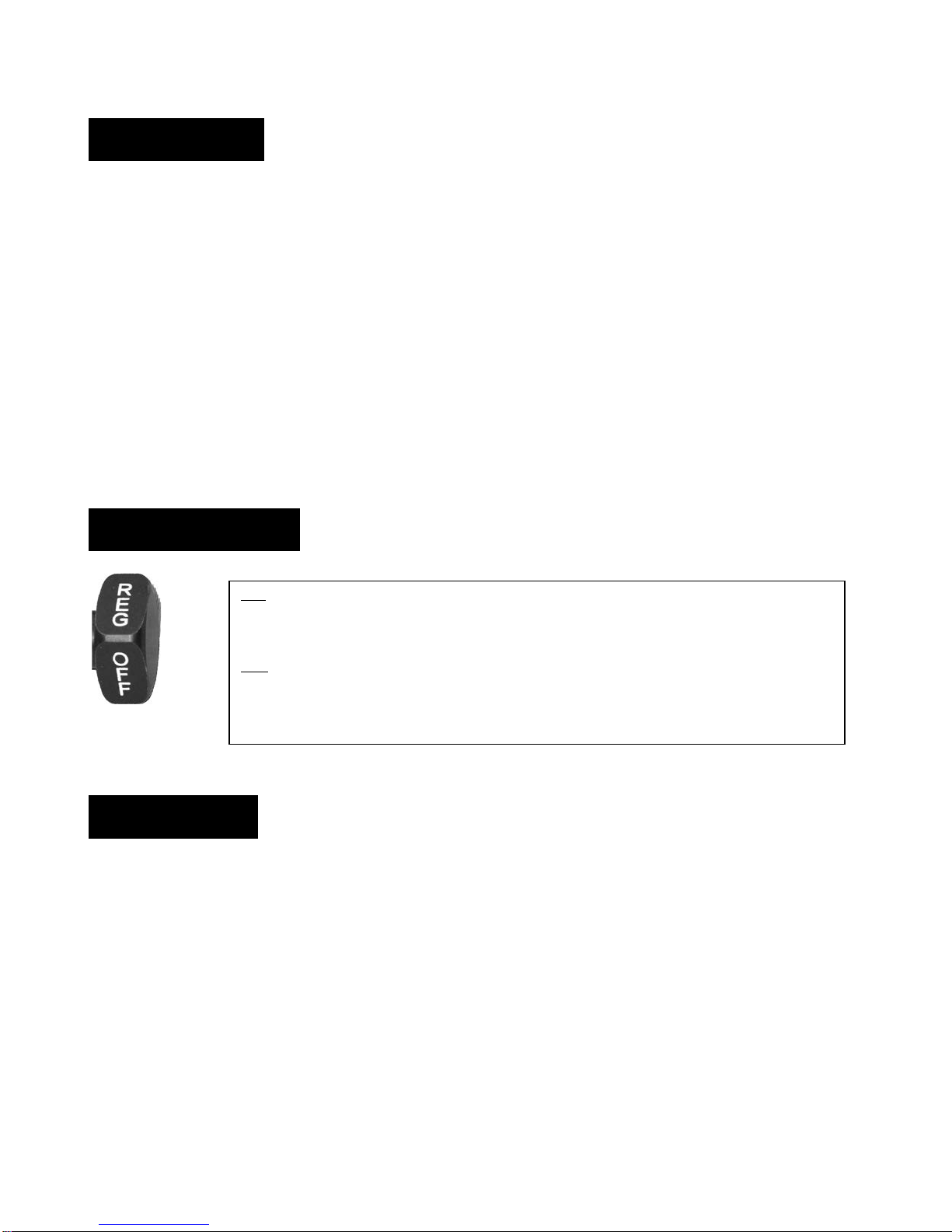
OFF Supply suction is off and patient circuit is vented to atmospheric pressure.
REG Supply suction is on and regulated output is controlled to prescribed setting.
Lo Spike: Accuracy of regulation depends primarily on the ability to provide a consistent level
of vacuum under changing flow conditions.
Involuntary pneumatic biopsy, or tissue damage, can occur when high levels of vacuum are
applied to delicate tissue. With a Boehringer regulator, you can depend on very low “spike”
compared to our competitor’s models.
"Spike" is the variation in indicated suction as flow in the collection circuit changes from an a
free flowing condition to an occluded condition. We measure spike as the change in indicated
suction from full flow to a no flow condition using a typical collection circuit with a 14 French
catheter. To test, set the regulator to 50 mmHg flowing, and then allow occlude the 14Fr
catheter. The change in the indicated suction level is "Spike".
Boehringer regulators are checked on the assembly line to meet a specification of less than
10% of the indicated setting, for example 5mmHg spike at a 50 mmHg setting.
An evaluation of a regulator’s spike allows one to determine whether the device is truly
“regulating”. A safe and reliable regulator should regulate to its set position regardless of
variable flow conditions.
PARALLAX Inaccuracy caused by observational position of an indicating element (pointer) to a reference
element (scale).
This product is intended for use by or on the order of a physician. It is to be used by individuals who are properly
trained in the use of Vacuum Assisted Venous Drainage during Cardiopulmonary Bypass.
The Boehringer VAVD Controller was designed to provide accurate control of wall suction from 15 - 60 mmHg
for use in vacuum assisted venous drainage and the removal of waste anesthetic gases in cardiac surgery. The
VAVD provides a redundant safety device that vents excess negative pressure > 95 mmHg and positive
pressure > 10 cmH2O.
Definition of Terms and Symbology
Indications for Use
3930.003 Rev C P/N 34031 3 of 9
Released July 2014 User Manual VAVD Controller

This device is designed and sold for use only as indicated
This product is intended for use by or on the order of a physician. It is to be used by individuals who are
properly trained in medical suctioning procedures. Please read these instructions carefully.
A High Flow Bubble Barb fitting is provided to connect the regulator to a central suction source. Braid
reinforced tubing must be used to preclude the possibility of suction tubing collapse during extended
periods of use. The hose supplying suction to the regulator should have a vertical orientation to reduce
the possibility of kinking over time. Any deformation of this tubing may be indicative of a reduction in
flow to the suction regulator and accompanying patient circuit.
Always verify the regulator is attached to an appropriate source of suction, and that suction is present,
before attaching a patient collection circuit. This can be verified by turning the control valve to REG, and
adjusting the control knob to increase suction. The presence of suction can be audibly heard from at the
patient port of the regulator.
Suction regulators must only be attached to vacuum systems. Do not attach to compressed air,
nitrogen, or oxygen sources.
Do not cover, obstruct, or occlude the inlet of the interrupter where it is labeled ‘Do Not Occlude Vent’.
Do not attempt to calibrate this safety device. Return to the factory for appropriate service.
When used in conjunction with a venous return reservoir for cardiac bypass surgery: ensure that all air
detection and control devices are in proper working order, have all field suction pumps clear and
operating and monitor and control the fluid levels in the reservoir to prescribed limits.
When used for the purpose of vacuum assisted venous drainage for cardiac surgery, ensure proper
placement of cannulae and verify that venous drainage is properly occurring.
Suction catheters, collection canisters and suction tubing must be carefully evaluated and selected to
ensure adequate function for the specific clinical environment and intended field of use.
Always verify regulator operation (Spike, see page 4 for details) before use on a patient. Verify
operation by establishing the desired vacuum level with the collection circuit and suction catheter
attached to the regulator. Occlude the suction catheter and note that the indicated vacuum does not
rise by more than 10% of the original setting.
The Suction Flowmeter of the 3930 uses the facility’s medical-surgical vacuum source for Waste
Anesthetic Gas Disposal (WAGD). As such, flammable anesthetics or other flammable vapors are
required to be diluted below the lower flammable limit prior to disposal into the medical-surgical vacuum
system per NFPA 99-2012 (5.1.3.8)
Safety Information
WARNING!
Contraindications
3930.003 Rev C P/N 34031 4 of 9
Released July 2014 User Manual VAVD Controller

Operation
FEATURES
Mode Selector Valve:
Square knob to easily switch
between operating modes
(OFF/REG).
Adjusting Knob:
Extra large, easy grip knob turns
COUNTER-CLOCKWISE (direction
of arrow) to increase suction setting
and CLOCKWISE to decrease
suction setting
Independent Safety Relief:
1. High Negativity Safety Vent:
Safety mechanism limits maximum suction to less
than 100mmHg.
2. Positive Pressure Relief:
Safety mechanism vents excess positive pressure.
WARNING! – Safety Port:
Do Not Occlude
Patented Linear Gauge:
Allows accurate readings from 180° field of view
and never requires calibration. Each range has
unique color-coding
Integral Supply Gauge:
Supply pressure drop allows
for quick identification and
correction of open caps or
kinks in tubing.
Adjustable Bracket:
Allows mounting to vertical
poles.
WAGD Suction Flowmeter:
For attachment to the scavenger
port on the membrane oxygenator
via the provided WAGD tubing with
safety vents.
DECREASE
INCREASE
3930.003 Rev C P/N 34031 5 of 9
Released July 2014 User Manual VAVD Controller

The VAVD regulator incorporates a 2-way selector valve for selecting no suction or a preset level of suction.
REG Mode: With the vacuum regulator attached to the vacuum system and a vacuum capable venous reservoir
attached to the vacuum regulator, rotating the control valve on the side of the vacuum regulator will place the
vacuum regulator in the REG mode. Vacuum can now be regulated from 15-60 mmHg or shut off by turning the
knob all the way clockwise.
With the control valve in the REG position, wall vacuum may be controlled to a specific level by turning the large
adjusting knob in the direction indicated. A spring opposed diaphragm assembly precisely controls the level of
suction provided at the lower inlet port of the regulator. This assembly senses changes in the venous return
circuit and makes appropriate adjustments to maintain the vacuum level that has been selected. Vacuum level is
adjusted by turning the large knob in the direction indicated. Regulated settings are verified with the large, easy
to read gauge.
OFF Mode: When the vacuum regulator is on the OFF position, the vacuum return circuit reverts to atmospheric
pressure.
The model 3930 regulator is supplied with a high flow bubble barb for attachment to wall suction. Only braid
reinforced tubing suitable for suction service should be used when connecting this to a central suction supply.
Inappropriate tubing could lead to a reduction in flow and compromise patient safety. The tubing should exit the
fitting in a downward vertical orientation to minimize the possibility of kinking the supply tubing over time.
Connect the externally reinforced clear tubing to the WAGD connection (below). This line is meant to conduct
waste gases without applying negative pressure to the blood or gas inlet of the oxygenator.
The patient connection must be connected to an appropriate cardiotomy reservoir, do not draw patient materials
directly into this line, the use of a disposable vapor trap between the suction regulator and the cardiotomy
reservoir is recommended.
INSTALLATION
THEORY OF OPERATION
3930.003 Rev C P/N 34031 6 of 9
Released July 2014 User Manual VAVD Controller
1. With the selector valve of the unit in the OFF position and the valve on the suction flowmeter fully closed
(clockwise), verify the supply gauge reads > 500 mmHg. If a minimum of 500 mmHg is not available,
check the incoming supply tubing, or check the suction inlet to confirm it is compliant with the NFPA 99
standard.
2. Adjust the WAGD suction flowmeter all of the way open (counterclockwise) verify the flowmeter
registers the flow and that audible suction can be heard. Ensure the supply gauge registers > 400
mmHg with the suction flowmeter all of the way open. If a minimum of 400 mmHg is not maintained,
VAVD may not be effectively applied, resulting in patient risk. Check the incoming supply tubing, or
check the suction inlet to confirm it is compliant with the NFPA 99 standard.
3. Connect the VAVD Controller to the cardiotomy reservoir. Turn the control valve to ON and adjust the
output to 20 mmHg. Ensure suction can be audibly heard and the unit maintains the 20 mmHg set point.
If the unit is unable to pass the pre-use criteria, please contact Customer Service 800-642-4945 to have
the unit returned for needed service / calibration.
Follow venous reservoir manufacturer’s instructions regarding proper set up and use.
Ensure that all air detection and control devices are in proper working order. It is recommended that over
pressurization and vacuum relief valves are used in conjunction with the venous reservoir. It is recommended
that the pressure inside the venous reservoir be monitored by the clinician.
Attach the externally reinforced clear tubing (WAGD Tube Assembly) to the vent port on the membrane
oxygenator. The tube is bidirectional and either end may be connected to the vent port as well as the WAGD
connection on Suction Flowmeter. Do not block or occlude the vent holes that are at both ends of the tube.
Follow membrane oxygenator manufacturer's recommendation for suction flow settings.
Connect the reservoir connection port to the venous reservoir. It is highly recommended to use a disposable
vapor trap between the vacuum regulator and the venous reservoir
OFF: With control valve in the OFF position, suction is off and the collection circuit is returned to
atmospheric pressure by an internal vent port, a special feature of the Boehringer design. Any additional
positive pressure or negative pressure in excess of 100mmHg will be vented from the patient collection
circuit.
REG: With control valve in the REG position, wall suction may be controlled to a specific level by turning
the large adjusting knob in the direction indicated. A spring opposed diaphragm assembly precisely
controls the level of suction provided at the lower port of the Regulator within the range of the gauge. This
assembly "senses" changes in the patient collection circuit and makes appropriate adjustments to maintain
the suction level that has been selected. Regulated settings are verified by the large, easy to read gauge.
MODE SELECTION
CLINICAL USE
PRE-USE CHECK
3930.003 Rev C P/N 34031 7 of 9
Released July 2014 User Manual VAVD Controller

Your VAVD Controller has been designed with the highest quality materials and to the strictest production
tolerances. Unlike common hospital suction regulators, the VAVD controller has precision low suction output
and redundant safety features to limit excess negative pressure and positive pressure. Given the critical nature
of the clinical interventions for which the VAVD Controller is employed, and the inherent risk involved in applying
unregulated suction during these procedures, only factory service and calibration may be performed on this unit.
Factory calibration is recommended - -
•Any time fluid has entered the VAVD Controller.
•Any time there is physical damage noted to VAVD Controller.
•Any time the VAVD Controller fails a pre-use test.
•A minimum of every 24 months.
WAGD Tube Assembly (Model 3947) - -
It is recommended to replace the WAGD Tube Assembly every (3) three months or sooner if it has any sign of
being damaged or visibly contaminated. Additional WAGD Tube Assemblies (Model 3947) are available for
purchase.
Please call Customer Service at 800-642-4945 to obtain an RMA prior to return or to order additional WAGD
Tube Assemblies (Model 3947). Once the unit has been received you will be contacted with an estimate of any
service charges. Units will be serviced within five days of receipt of charge authorization.
After patient use, wipe all exterior surfaces of the VAVD Controller with an appropriate surface
disinfectant. Appropriate disinfectants are:
•3M Quat®
•Cavacide®
Your VAVD regulator has been designed for years of trouble-free service. Should you experience difficulty that
is not the result of damage to the instrument, the most likely cause is aspiration of dirt and/or fluids into the
Regulator.
Symptom Probable Cause Solution
Instrument fails to provide suction at
the patient port.
The supply or patient fittings are clogged,
or the incoming suction tubing is collapsed
or kinked.
Replace or clean the fittings. Replace the
incoming suction line.
Gauge doesn't respond to changes in
suction (via control valve or
adjustment knob)
Gauge diaphragm is improperly sealed on
the gauge piston and/or view tube Reference Boehringer Tech Bulletin
3700.044.
Gauge piston is discolored. Material has entered the inside of the
device. Instrument is contaminated. Please return to
the factory for service.
Instrument will not shut off or exhibits
high spike. Dried fluids may have cut the quad ring
seal. Please return to the factory for service.
Instrument fails to regulate suction Piston/Stem surface is binding with foreign
matter Please return to the factory for service.
Audible sound coming from safety
port on safety interrupter Material has entered the inside of the
device. Instrument is contaminated. Please return to
the factory for service.
TROUBLESHOOTING
MAINENANCE
Cleaning & Disinfection
3930.003 Rev C P/N 34031 8 of 9
Released July 2014 User Manual VAVD Controller

•Inlet and outlet fittings: 1/8 NPT, High Flow Bubble Barb (P/N 2469)
•Gauge accuracy ANSI Class B, ± 5% FS (± 3 mmHg)
•Regulation Accuracy: ±10% FS from full flow to zero flow with 14 FR catheter attached.
•Leak rate in OFF position: less than 1 cc/min
•Materials: polycarbonate, hard-anodized aluminum, stainless steel, Buna rubber, acetal copolymer.
Model
Regulation Range
User Selectable Modes
Wt. (lb)*
H x W x D (in)
3930 15 - 60 mmHg Off & Regulated Control 3.40 lbs 101/4” x 7½” x 81/2”
Operating and Storage Limits
We recommend that Boehringer Suction regulators be operated and stored at controlled conditions that typically
reflect the medical facility environment.
Boehringer Laboratories, Inc. guarantees your VAVD regulator for FIVE years from the date of manufacture.
Boehringer Laboratories, Inc. warrants to the original purchaser, new suction regulators purchased directly from
Boehringer Laboratories, Inc. or from an authorized dealer or representative. This warranty guarantees the
suction regulators to be free from functional defects in materials and workmanship. We also guarantee that our
suction regulators will meet our published specifications.
All regulators returned for repair shall be clean and free from contamination prior to shipment. This requirement
is for the safety of our employees as well as to comply with Federal Law prohibiting the shipment of unmarked
biohazard materials. If units are returned contaminated, a cleaning charge may result.
A service charge may be assessed on any unit returned that shows evidence of gross abuse.
Boehringer Laboratories, Inc. is the only authorized warranty service center for your VAVD regulator.
This warranty excludes acts of God, fire, flood and acts of war, terror or insurrection.
Boehringer Laboratories’ sole and exclusive remedy under this warranty is limited to repairing and/or replacing
the suction regulator. There are no other express or implied warranties beyond these warranties set forth above.
At Boehringer Laboratories, we are committed to lowering your suction regulator costs of operation!
A Return Material Authorization Number (RMA) must be obtained prior to returning a unit for service. Please
contact Customer Service at
SPECIFICATIONS
Warranty and Repair
Boehringer Laboratories, LLC
800-642-4945
info@boehringerlabs.com
300 Thoms Dr
Phoenixville, PA 19460
www.boehringerlabs.com
Covered under one or more of the following Boehringer patents (Additional Patents Pending):
6,264,890 6,228,056 5,992,239 5,879,624 5,409,491 5,372,593
5,354,262 5,203,778
2011 Boehringer Laboratories, Inc.© Boehringer is a trademark of Boehringer Laboratories, Inc.
3930.003 Rev C P/N 34031 9 of 9
Released July 2014 User Manual VAVD Controller
Table of contents
Other Boehringer Laboratory Equipment manuals