INDEX
INDEX.......................................................................................................................................................................................................3
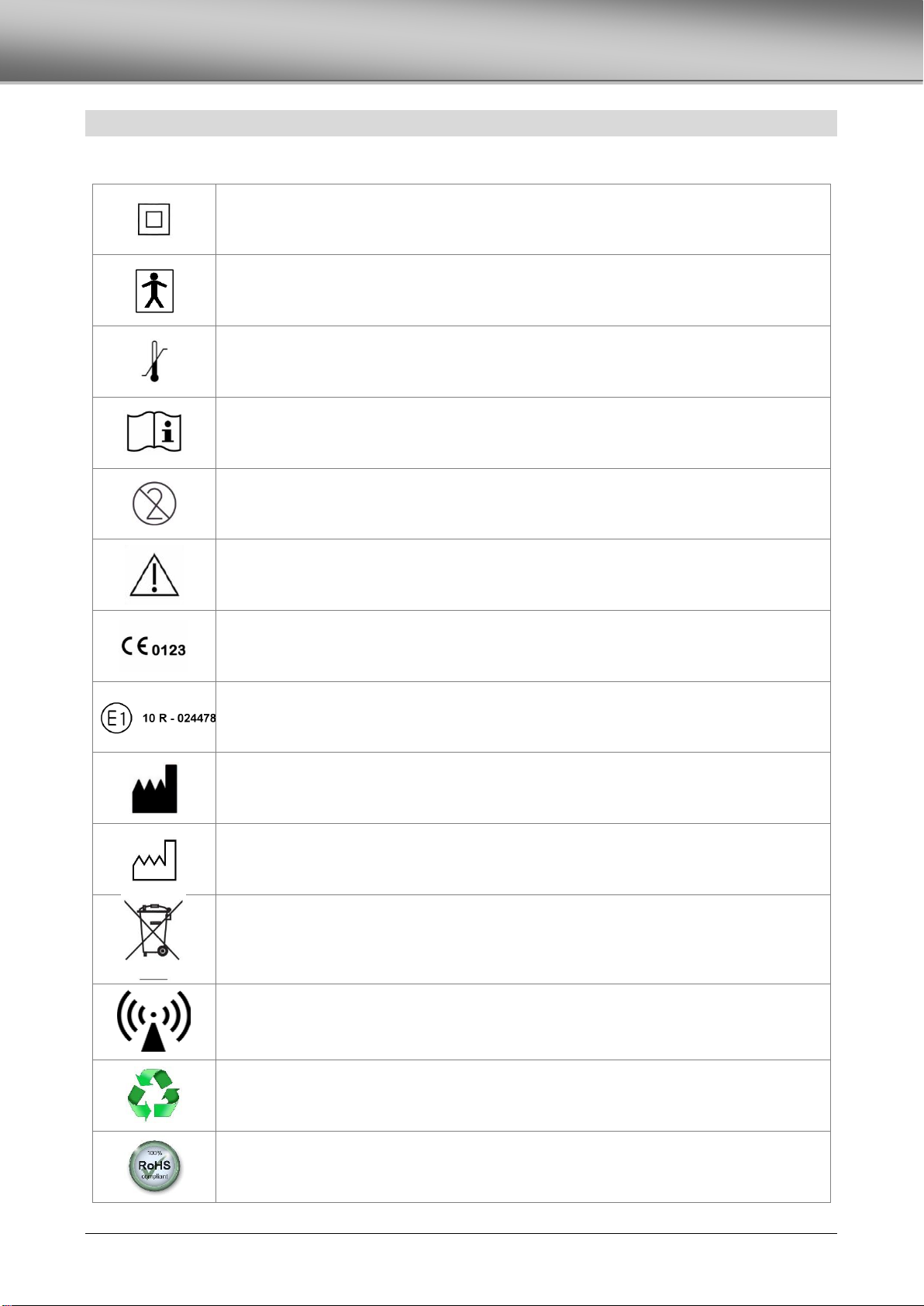
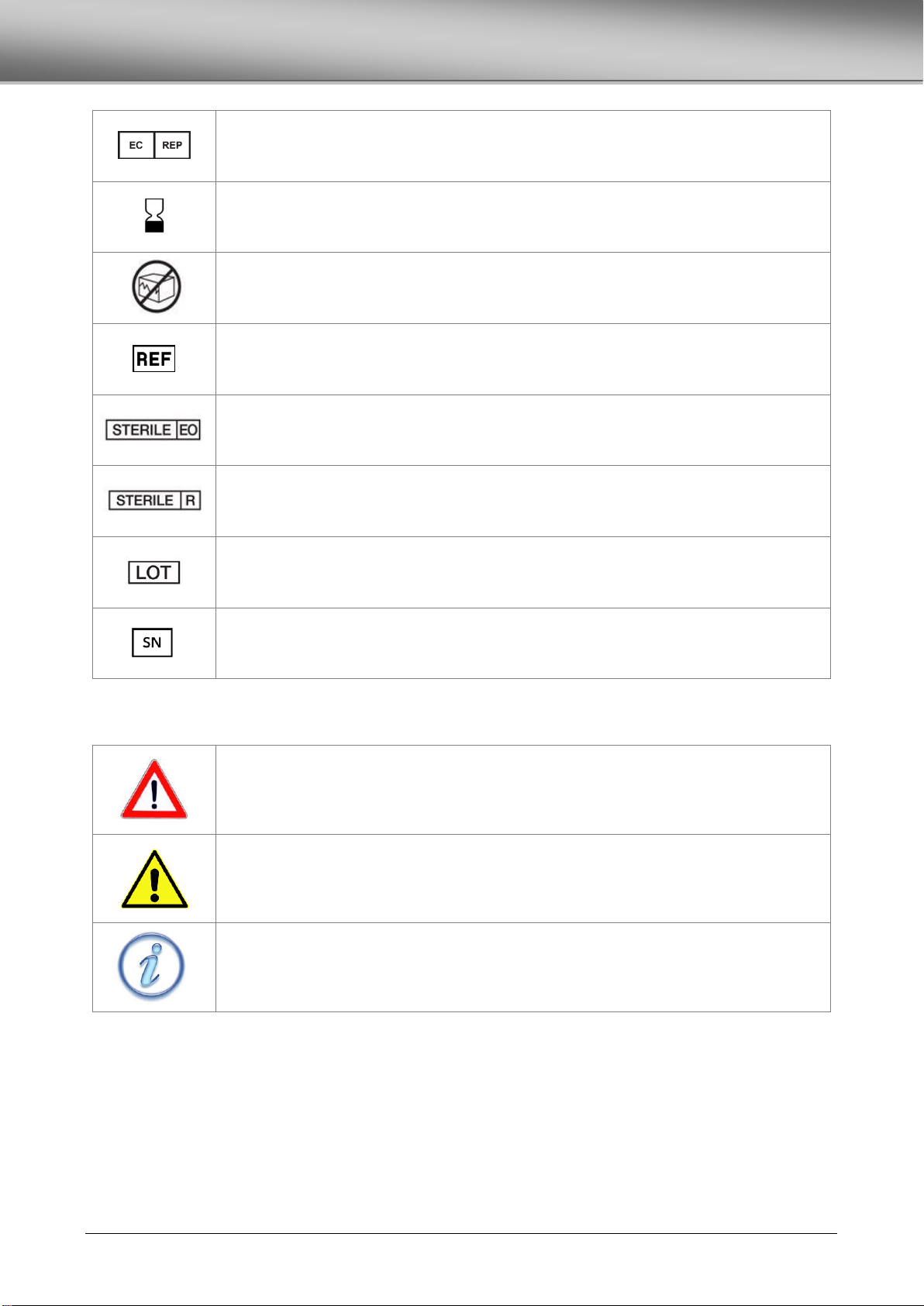
SYMBOLS .................................................................................................................................................................................................4
WARNINGS, PRECAUTIONS AND IMPORTANT INFORMATION................................................................................................................6
IMPORTANT INFORMATION....................................................................................................................................................................7
OB500 SUCTION UNIT..............................................................................................................................................................................8
DESCRIPTION AND INTENDED USE STATEMENT ................................................................................................................................8
CONTRA INDICATION FOR USE...........................................................................................................................................................8
CONTROLS, INDICATIONS AND CHECK PANEL....................................................................................................................................8
CONTROL MODULE ............................................................................................................................................................................9
SUCTION UNIT....................................................................................................................................................................................9
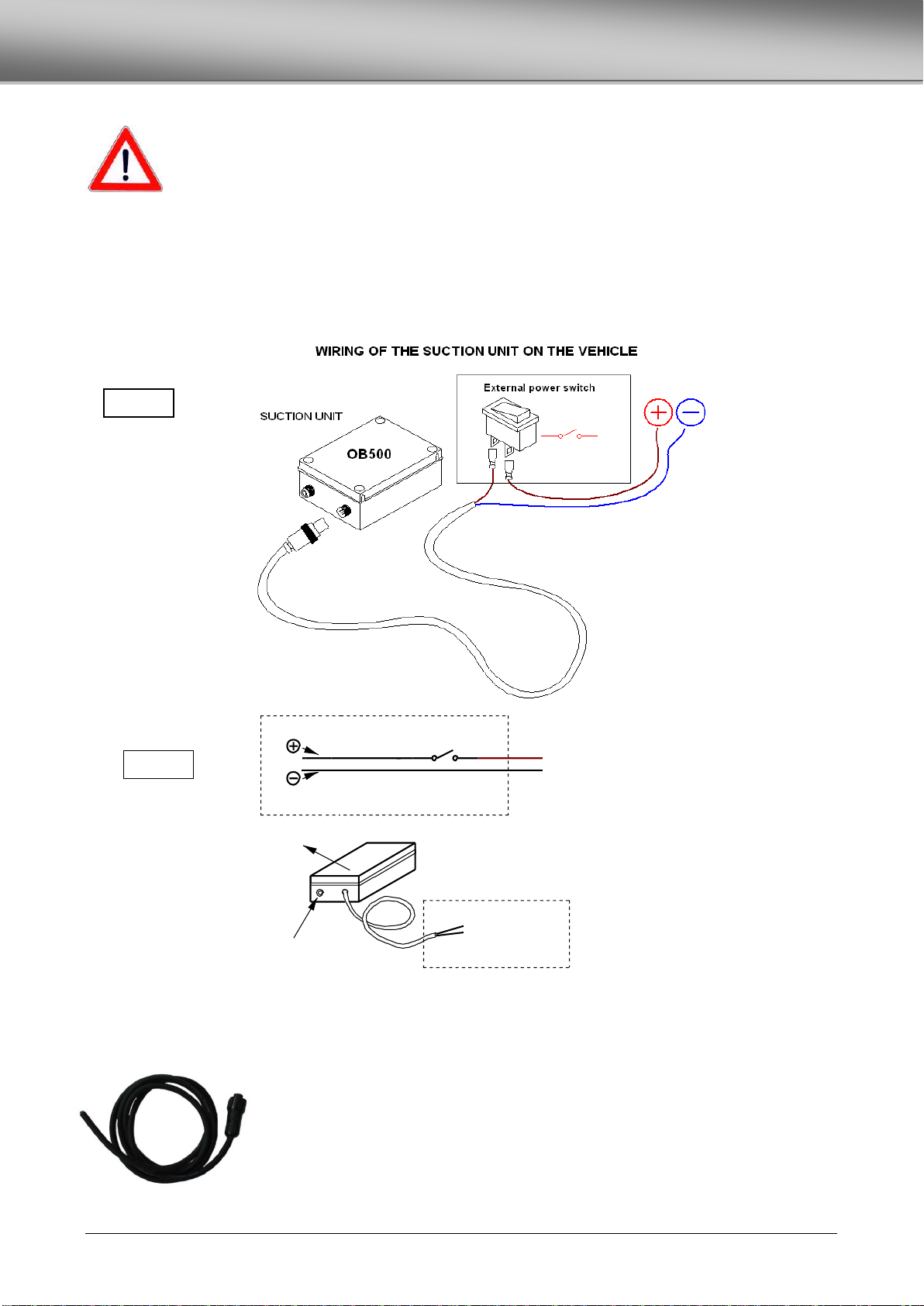
ELECTRICAL CONNECTIONS..............................................................................................................................................................10
TEST OF THE UNIT ............................................................................................................................................................................11
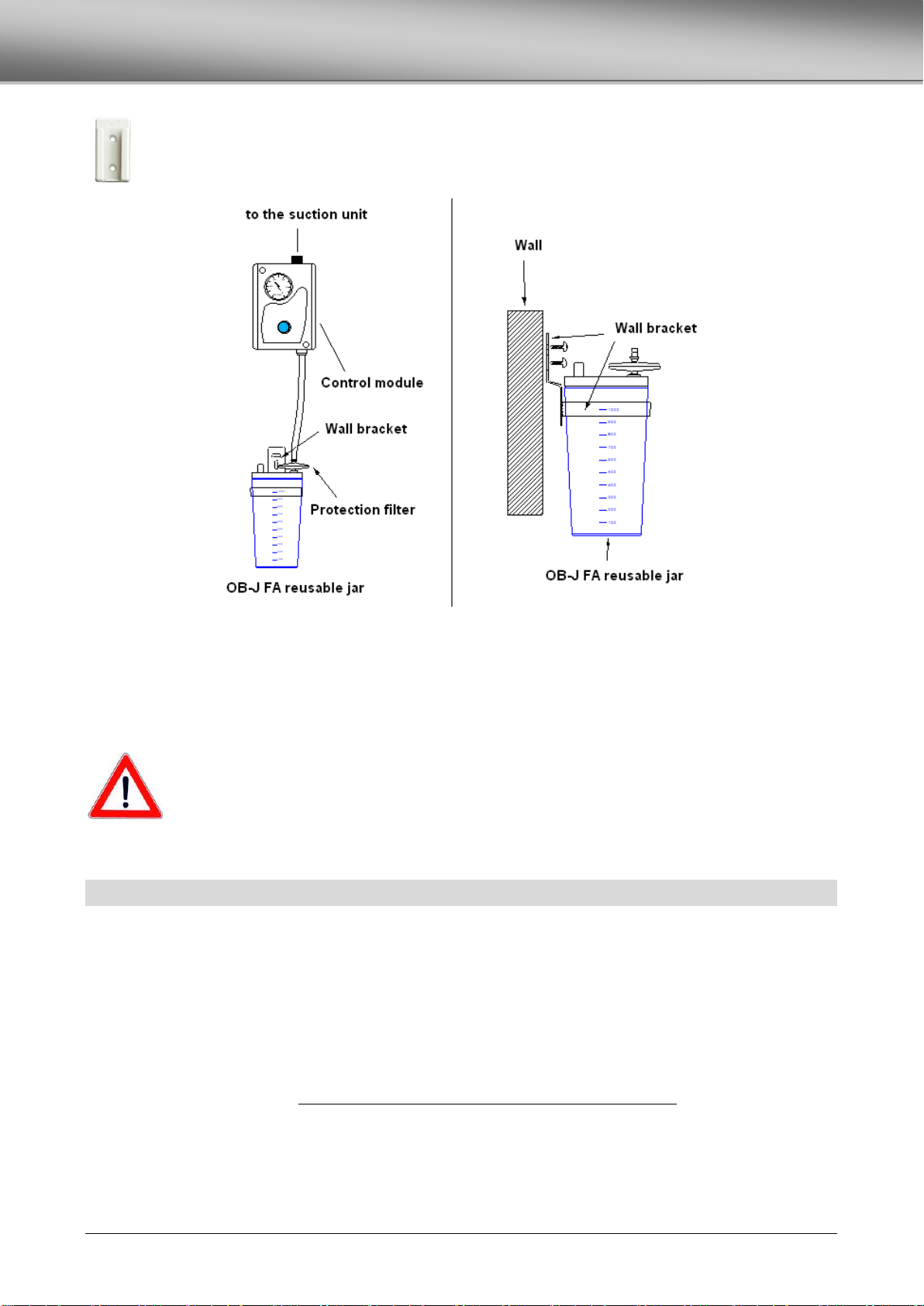
COLLECTION JAR...............................................................................................................................................................................11
OB-J FA REUSABLE, AUTOCLAVABLE JAR..........................................................................................................................................11
PROTECTION FILTER .........................................................................................................................................................................11
REUSABLE JAR WITH DISPOSABLE LINER SERRES® ...........................................................................................................................12
OB-J COLLECTION JAR.......................................................................................................................................................................12
JANKAUER SUCTION TUBE AND FINGER-TYP END-PIECE .................................................................................................................12
WALL BRACKET FOR THE JAR............................................................................................................................................................12
POWER SUPPLY ................................................................................................................................................................................13
MAINTENANCE AND REUSE...................................................................................................................................................................13
AFTER EACH REUSE ..........................................................................................................................................................................13
CLEANING INSTRUCTION..................................................................................................................................................................14
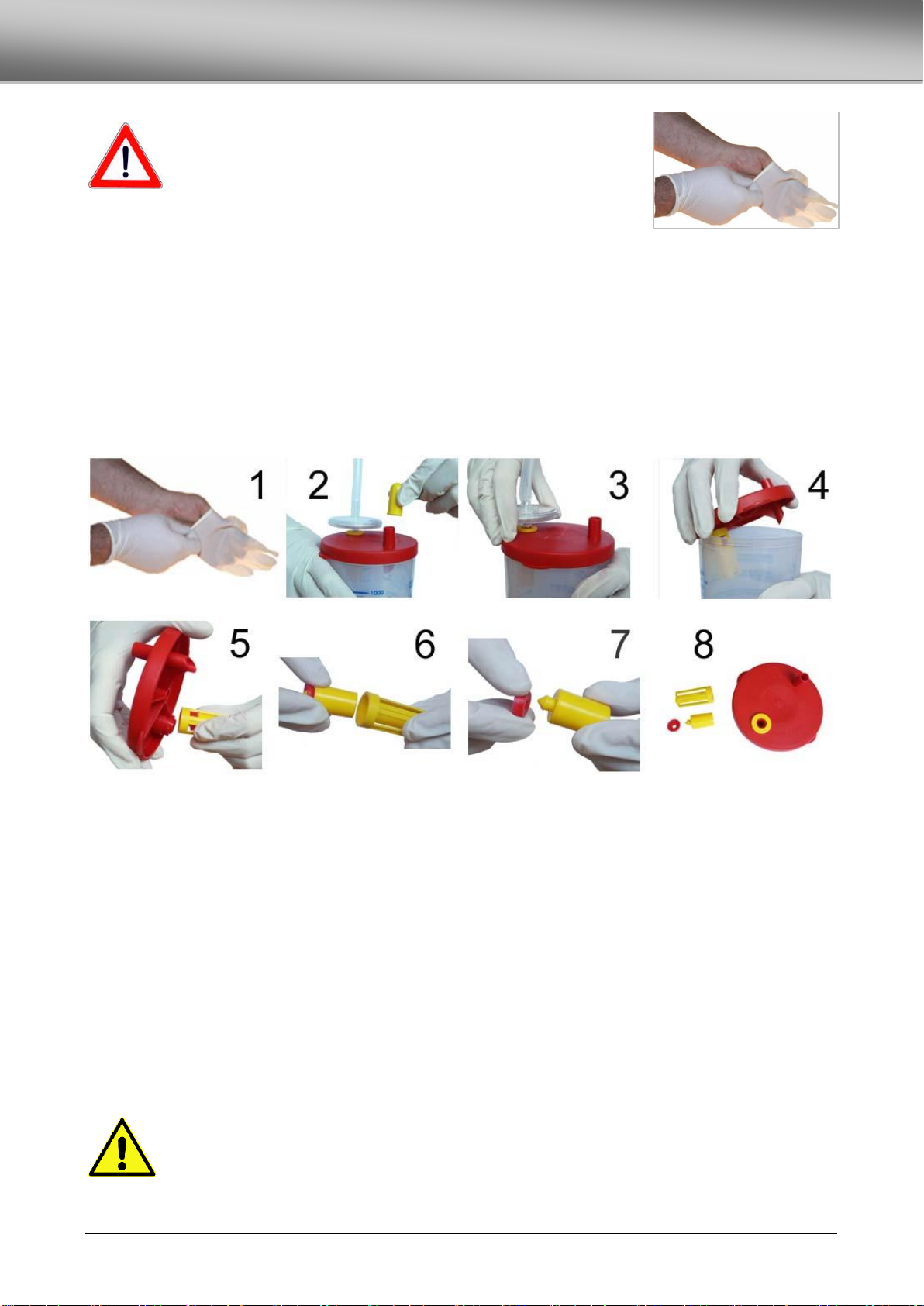
REUSABLE COLLECTION JAR OB-J FA ................................................................................................................................................14
DECONTAMINATION OF THE COLLECTION JAR ................................................................................................................................14
REPLACING THE PROTECTION FILTER...............................................................................................................................................15
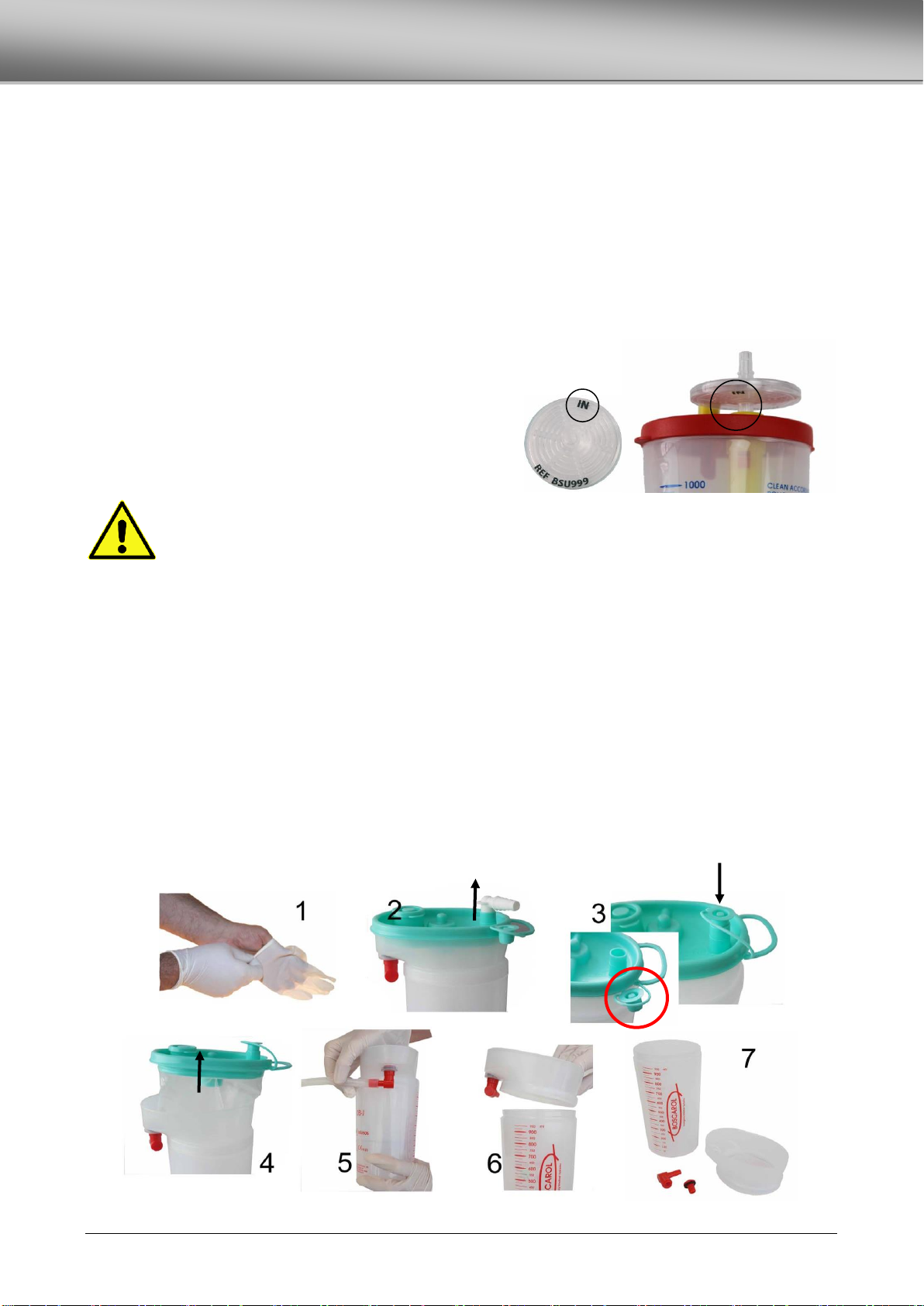
REUSABLE COLLECTION JAR OB-J .....................................................................................................................................................15
REUSABLE COLLECTION JAR SERRES®...............................................................................................................................................16
REASSEMBLY OF THE JAR .................................................................................................................................................................16
DISPOSAL OF CONTAMINATED PARTS..............................................................................................................................................17
CLEANING THE SUCTION UNIT .........................................................................................................................................................17
SAFETY..............................................................................................................................................................................................17
DISPOSING OF THE SUCTION UNIT...................................................................................................................................................17
ACCESSORIES AND SPARE PARTS...........................................................................................................................................................18
SERVICE..................................................................................................................................................................................................19
FAULT FINDING......................................................................................................................................................................................19
TECHNICAL DATA AND CONFORMITY TO INTERNATIONAL LAW...........................................................................................................19
THE RISKS OF RECIPROCAL INTERFERENCE WITH OTHER DEVICES........................................................................................................21
RISK OF ELECTROMAGNETIC INTERFERENCE AND POSSIBLE REMEDIES ...............................................................................................21
GUARANTEE...........................................................................................................................................................................................24
DECLARATION OF CONFORMITY............................................................................................................................................................25