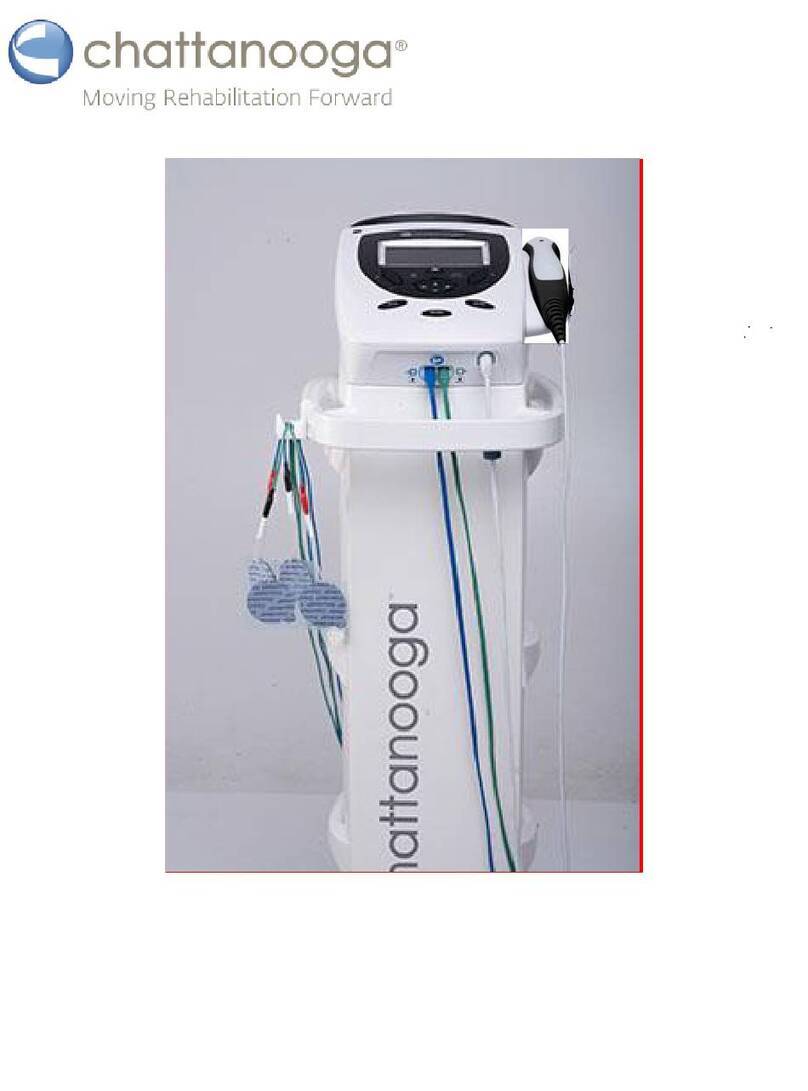
4
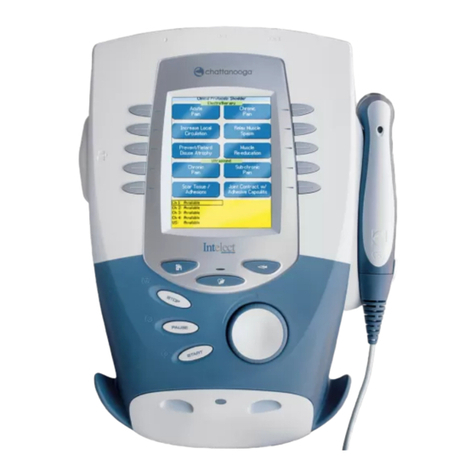
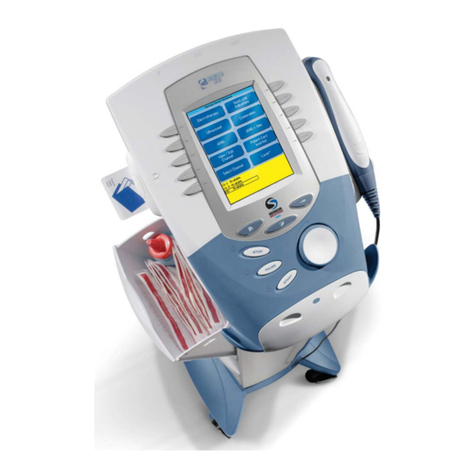
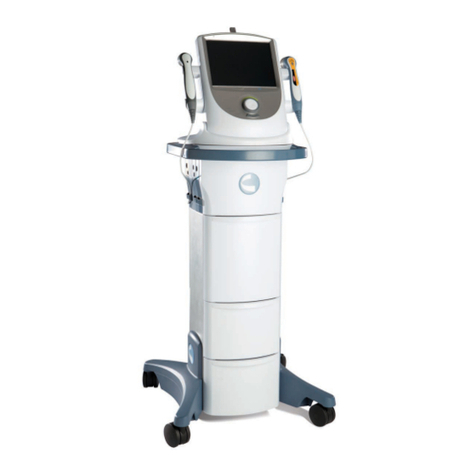
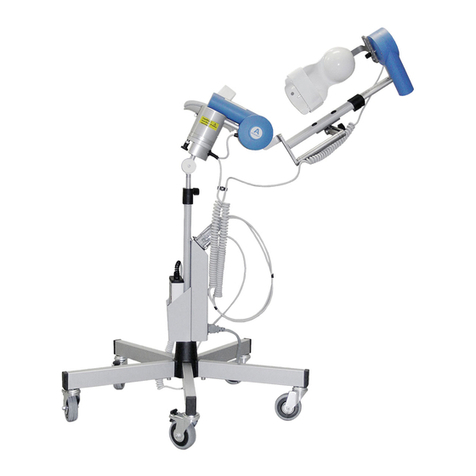
Neo Stimulation Channel 1 & 2 + EMG Module Manual
• This unit should be operated at 10°C to 45°C and 0% to 90% Relative Humidity.
The unit should be transported and stored at 0°C to 60°C and 0% to 95% Relative
Humidity.
• Device is designed to comply with electromagnetic safety standards. This
equipment generates, uses, and can radiate radio frequency energy and, if not
installed and used in accordance with instructions, may cause harmful
interference to other devices in the vicinity. However, there is no guarantee that
interference will not occur in a particular installation. Harmful interference to
other devices can be determined by turning this equipment on and off. Try to
correct the interference using one or more of the following:
- Reorient or relocate the receiving device
- Increase the separation between the equipment
- Connect the equipment to an outlet on a different circuit from that to
which the other device(s) are connected and consult the factory field
service technician for help.
- Consult your authorized DJO dealer for help.
• Do not operate this unit when connected to any unit other than DJO devices or
accessories specifically described in user or service manuals.
• DO NOT disassemble, modify, or remodel the unit or accessories.This may cause
unit damage, malfunction, electrical shock, fire, or personal injury.
• DO NOT permit foreign materials, liquids or cleaning agents to enter the unit,
including, but not limited to, inflammables, water, and metallic objects from
entering the unit, to prevent unit damage, malfunction, electrical shock, fire, or
personal injury.
• If you have difficulty operating the unit after carefully reviewing this user
manual, contact your DJO dealer for assistance.
DANGER
CAUTION
WARNING
• Stimulus delivered by theTENS waveforms of this device, in certain
configurations, will deliver a charge of 25 microcoulombs (μC) or
greater per pulse and may be sufficient to cause electrocution.
Electrical current of this magnitude must not flow through the
thorax because it may cause a cardiac arrhythmia.
• Besuretoreadallinstructionsforoperationbeforetreatingpatient.
• Makecertaintheunitiselectricallygroundedbyconnectingonlyto
a grounded electrical service receptacle conforming to the applicable
national and local electrical codes.
• Caremustbetakenwhenoperatingthisequipmentaroundother
equipment. Potential electromagnetic or other interference could occur
to this or to the other equipment. Try to minimize this interference by not
using other equipment in conjunction with it.
• ThesafetyofTENSwaveformsforuseduringpregnancyorbirthhasnot
been established.
• TENSisnoteffectiveforpainofcentralorigin.(Thisincludesheadache.)
• TENSwaveformshavenocurativevalue.
• Electronicmonitoringequipment(suchasECGmonitorsandECGalarms)
may not operate properly when electrical stimulation is in use.
• TENSisasymptomatictreatment,andassuch,suppressesthesensation
of pain which would otherwise serve as a protective mechanism.
• Contaminatedelectrodesandleadwirescanleadtoinfection.
• Useofelectrodewithdegradedhydro-gelcanresultininfectiontothe
skin burn.
• Useofelectrodeonmultiplepatientscanleadtoinfection.
• Stoptreatmentimmediatelyifpatientexperiencesdiscomfortorpain.
• Poweredmusclestimulatorsshouldbeusedonlywiththeleadsand
electrodes recommended for use by the manufacturer.
• Intheeventofall300-Levelora200-Levelerrormessagethatcannotbe
resolved, immediately stop all use of the system, and contact the dealer
or DJO for service. Errors andWarnings in these categories indicate an
internal problem with the system that must be tested by DJO or aTrained
Technician before any further operation or use of the system.
- Use of a system that indicates an Error or Warning in these categories
may pose a risk of injury to the patient, user, or extensive internal
damage to the system.
• DONOTconnecttheunittoanelectricalsupplywithoutfirstverifyingthat
the power supply is the correct voltage. Incorrect voltage may cause unit
damage, malfunction, electrical shock, fire, or personal injury. Your unit
was constructed to operate only on the electrical voltage specified on the
Voltage Rating and Serial Number Plate.
- Contact your DJO dealer if the unit is not properly rated.
• Keepelectrodesseparatedduringtreatment.Electrodesincontactwith
each other could result in improper stimulation or skin burns.
• Longtermeffectsofchronicelectricalstimulationareunknown.
• Stimulationshouldnotbeappliedovertheanteriorneckormouth.
Severe spasm of the laryngeal and pharyngeal muscles may occur and the
contractions may be strong enough to close the airway or cause difficulty
in breathing.
• Stimulationshouldnotbeappliedtransthoracicallyinthatthe
introduction of electrical current into the heart may cause cardiac
arrhythmia.
• Stimulationshouldnotbeappliedoverswollen,infected,andinflamed
areas or skin eruptions, e.g., phlebitis, thrombophlebitis, varicose veins,
etc.
• Stimulationshouldnotbeappliedover,orinproximityto,cancerous
lesions.
• Electrotherapyoutputcurrentdensityisrelatedtoelectrodesize.Improper
application may result in patient injury. If any question arises as to the
proper electrode size, consult a licensed practitioner prior to therapy
session.
• Outputcurrentdensityisrelatedtoelectrodesize.Improperapplication
may result in patient injury. If any question arises as to the proper
electrode size, consult a licensed practitioner prior to therapy session.