Clement Clarke International MEDIX AC2000 User manual

AC2000, Econoneb & Turboneb 2
1639
AC2000
Econoneb/Turboneb 2
2. 6.
3.
4.
5.
1.
7.
1.
2.
6.
4.
5.
3.

Index
English - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Page 1
Français - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Page 13
Español - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Página 25
Deutsch - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Seite 37
Nederlands - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Pagina 49
Dansk - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Side 61
hrvatski jezik - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Stranica 73
slovenski (jezik) - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Stran 85


1
en
Contents
1. Product Identification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 2
2. Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 2
3. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 2
3.1 Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 2
4. Starter Kit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 3
5. Notes on Compressor Nebuliser Care . . . . . . . . . . . . . . . . . . . . . . . Page 3
6. Operating Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 3
6.1 Filling the Medication Chamber . . . . . . . . . . . . . . . . . . . . . . . . Page 4
6.2 Administrating the Medication . . . . . . . . . . . . . . . . . . . . . . . . . Page 4
6.3 Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 5
6.4 Changing the Attachments . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 5
6.5 Replace Filters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 5
6.6 Replace Fuse . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 6
6.7 Servicing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 6
7. Safety Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 6
8. Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 7
9. Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 7
10. Particle Size Distribution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 8
11. Guarantee . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 9
12. EMC (Electromagnetic Compatibility) Declaration . . . . . . . . . . . . . Page 10
13. Disposal Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 11
14. Spares and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 11
2
en
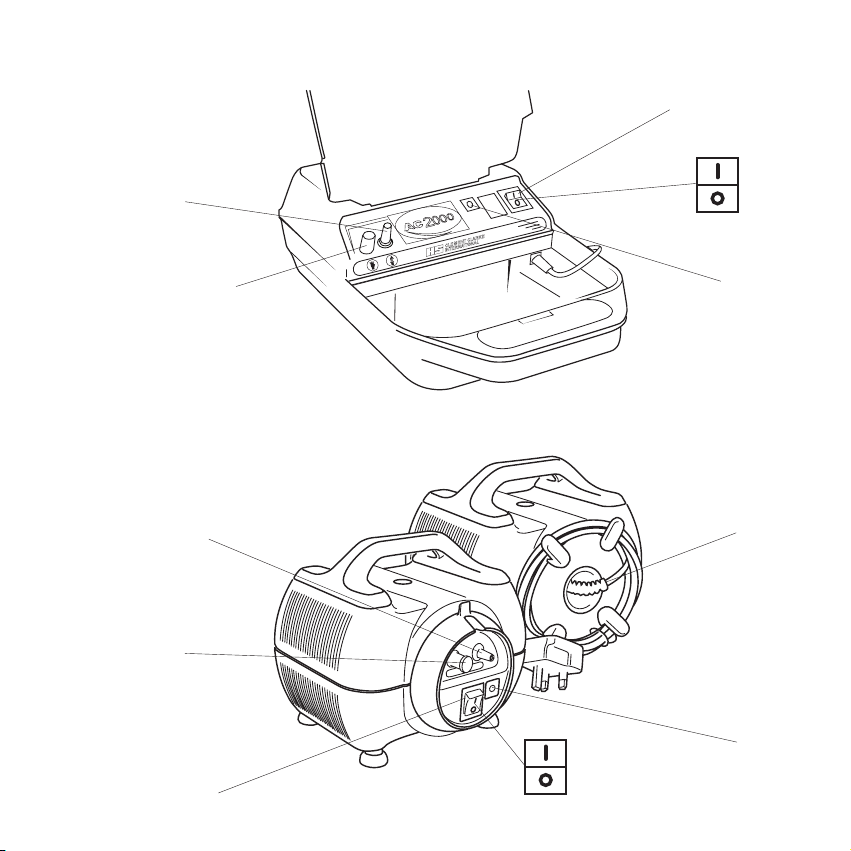
1. Product Identification
1. Outlet filter 5. Off
2. Inlet filter 6. Fuse (T1AL 250V)
3. Mains switch 7. Hard wired lead
4. On
2. Symbols
Consult Instructions for Use Fuse (T1AL 250V)
Caution Class II Equipment
Do not allow liquid to enter
mains input socket Type BF Equipment
Inlet filter Do not dispose of with household refuse
Outlet filter
3. Introduction
These compressor nebuliser systems are designed to deliver a fine aerosol mist from the liquid medication prescribed
by your doctor to treat your respiratory condition.
Use according to the instructions given by your doctor or physician.
Please read the instructions carefully to obtain the maximum benefit from the compressor nebuliser systems.
The compressor nebuliser systems are approved for the following environmental / ambient conditions for
transportation, use and storage purposes:
3.1Storage
The machine should be stored in a dust and smoke-free area.
Recommended: -25˚C - +70˚C
RH: 15% - 90%
Pressure: 700hPa - 1060hPa
WARNING: Do not use compressor nebuliser systems near active high frequency surgical equipment or
electro-magnetic equipment.

3
en
4. Starter kits are supplied with the AC2000 and Econoneb containing:
1 x medication chamber 1 x driveline
1 x inlet filter 1 x filter spanner
1 x mouthpiece 1 x adult face mask
1 x child face mask
The Turboneb 2 is not supplied with a starter kit.
The Turboneb 2 supplies the highest flow rate for nebulising viscous antibiotics and has been designed to operate
from mains voltage. Two versions are available, UK and EURO, (i.e. plug type) both 220-240V /50Hz.
This compressor-only unit provides flexibility for the user to choose the most appropriate accessories for
demanding applications.
Note:
•All MEDIX compressor nebulisers are continuously rated for repetitive use.
Please refer to the information label on the machine to establish that you have the correct voltage for your
mains supply.
• The parts listed above are required for correct function of these compressor nebulisers and must be in
compliance with EN 13544-1.
5. Notes on Compressor Nebuliser Care
Caution
1. Do not immerse machine in water.
2. Physician prescribed use only.
3. Never use more frequently than prescribed.
4. If therapy is having no effect, consult physician.
6. Operating Instructions (Mains Operated)
Operating Temperature:
Recommended: +5˚C - +40˚C
RH: 15% - 90%
Pressure: 700hPa - 1060hPa
1. Carefully withdraw compressor nebuliser from the box.
2. Connect mains lead to a mains power supply.
3. Connect driveline to outlet filter nozzle.
4. Connect nebuliser chamber, followed by mouthpiece or face mask, to the driveline.
5. Press mains switch on ( I ). The switch will illuminate, check airflow before each use.
6. The equipment can now be operated.

4
en
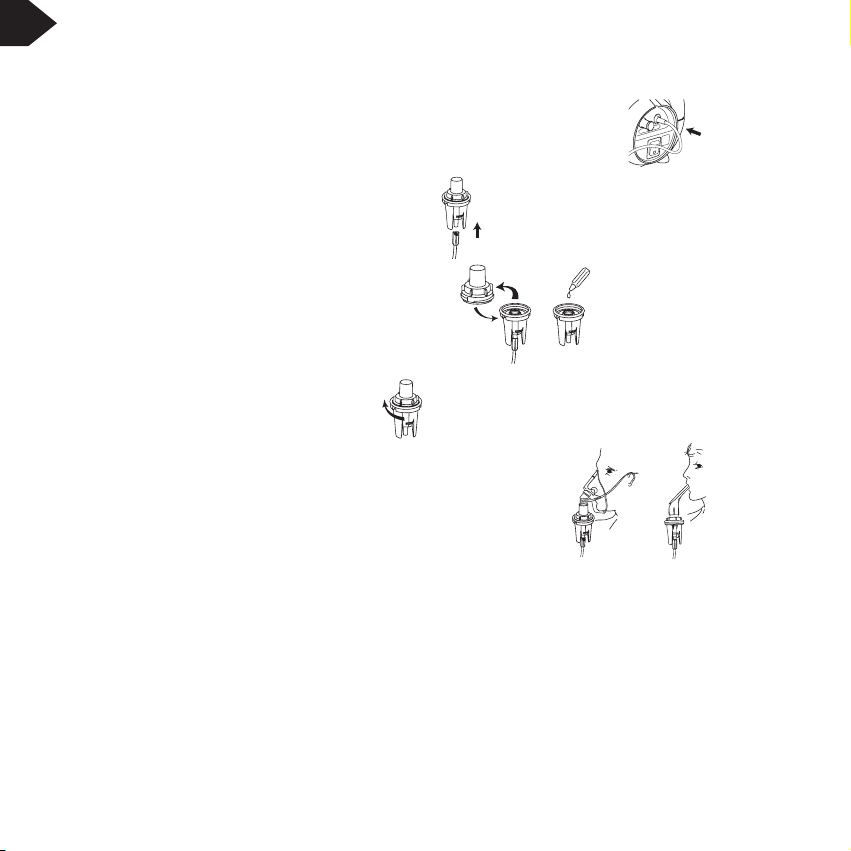
6.1Filling the Medication Chamber
For Single Patient Use
1. Connect one end of the driveline to the outlet filter on your compressor nebuliser.
2. Connect the other end of the driveline to the bottom of the blue medication chamber.
3.
Remove the medication chamber top from the cup and pour
medication into the chamber
(max. 10ml.).
4. Screw the top back onto the
medication chamber
.
5. Connect either the face mask or mouthpiece to the
medication
chamber
top.
All accessories must comply with EN 13544-1
6.2Administering the Medication
Switch the compressor unit on. Sitting in an upright relaxed position, place mouthpiece into the mouth (or face mask
over the nose and mouth) and start breathing slowly and deeply. Do not try to breathe quickly. If you have any problems
seek instruction from your doctor or physiotherapy department at your hospital.
Switch off the compressor nebuliser when the misting has stopped. Unplug the mains lead.

5
en
6.3Cleaning
In order that you benefit fully from your equipment, it is important that the machine and accessories are kept in a clean
condition. Ensure that the medication chamber is taken apart and washed in warm soapy water. Rinse and allow to dry
after each use. The jet holes should be blown clear by attaching the chamber to the air outlet from the compressor and
running the machine for a couple of minutes.
We advise that the tube is disconnected from both the outlet filter and the chamber after each use to prolong the life of
the connector.
The compressor nebuliser devices are suitable for cleaning daily with approved CE marked disinfectant wipes for the
lifetime of the device.
WARNING: Accessories are not for multiple patient use due to risk of infection.
Device must be cleaned between patients to prevent risk of infection.
6.4Changing the Attachments
It is recommended that the following disposable items should be changed as follows:
Medication chamber }
Inlet filter
Driveline change every 3 months
Face Mask
Mouthpiece }
Outlet filter change every 12 months
Only medication chambers supplied or approved by Clement Clarke International should be used.
6.5Replace filters
AC2000 - only replace inlet filter
Inlet filter:
Using the filter spanner, unscrew your used, white, inlet filter in an anti-clockwise
direction and discard.
Replace with a new filter, ensuring you have the white inlet filter.
Screw the white inlet filter, into the left-hand hole on the front panel, in a clockwise
direction, tightening with the filter spanner.
Outlet filter for the Econoneb/Turboneb 2:
Using the filter spanner, unscrew your used, blue, outlet filter in an anti-clockwise
direction and discard.
Replace with a new filter, ensuring you have the blue outlet filter.
Screw the blue outlet filter, into the right-hand hole on the front panel, in a clockwise
direction, tightening with the filter spanner.

6
en
6.6Replace Fuse
1. Switch off at mains.
2. Using a screwdriver or a coin, push and twist fuse in an anti-clockwise direction to extract fuse.
3. Replace with new fuse.
6.7Servicing
In order to maintain your warranty, your unit is required to be serviced by an approved service agent every 12 months.
Please contact the manufacturer or distributor for details of your nearest authorised service agent.
Warning: Disconnect power supply before servicing. When servicing, use only identical replacement parts.
Not suitable for use with flammable anaesthetics.
Caution: Do not immerse in water.
Please Note: When returning your nebuliser for servicing to Clement Clarke International, please contact
customer services for an authorised returns number.
Please do not return your consumables when returning your nebuliser for servicing.
The compressor nebuliser case should be wiped clean with an approved CE marked disinfectant wipe, before
returning for servicing.
7. Safety Precautions
• Always disconnect from electricity supply before undertaking any maintenance or cleaning.
• Never operate the unit where it may come into contact with water.
Should this happen, switch off at mains and unplug immediately.
• Never block air intake grills on the carrying case.
• Always keep electrical leads clear of heated surfaces.
• Position the unit on a clean surface. Do not place on carpet where fibres can be drawn into the unit when in use.
• Avoid using in a room where people smoke.
• Store in a clean, dry, dust free environment at room temperature.
• Not suitable for use with flammable anaesthetic gases.
• Do not modify the equipment or its accessories in any way.
• Do not allow liquid to enter the mains input socket.
• Performance information provided is in accordance with EN 13544-1 and may not apply to drugs in suspension or of
high viscosity form as there is a risk of particle size distribution curve being altered.
Please consult drug suppliers for further information.
• Accessories should not be shared, due to risk of infection.
• Not suitable for use in anaesthetic breathing systems or lung ventilator breathing systems.
Note: Refer to website for updates to IFU.
7
en
8. Troubleshooting
Problem Action
Air compressor will not If the green light in the mains switch is on, yet the motor does not function,
operate when switched on. there is an internal fault. The machine should be returned to the point of
purchase or an authorised person for examination.
If the green light(s) on the switches are off:
1. Check mains lead is securely connected each end.
2. For machines with a 3 pin plug (UK) remove and check the fuse in the plug.
Fuse - 3.15AL 25mm long.
3. Check mains fuse in the control panel. Fuse - T1AL 250V, 20mm long.
4. Disconnect unit from electricity supply. Check mains fuse in the mains
inlet socket.
Nebuliser chamber bubbles 1. Check inlet filter. Replace if necessary.
or produces little or no mist. 2. Wash nebuliser chamber. Replace if problem still occurs.
Time taken to nebulise 3. Check driveline tubing is not kinked or leaking. Replace if necessary.
significantly increases. 4. Service compressor.
9. Technical Specifications
AC2000 Econoneb Turboneb 2
Country UK/EU UK/EU UK/EU
Operating Voltage 220V-240V / 50Hz 220V-240V / 50Hz 230V / 50Hz
Power Consumption 62VA (260mA) 64VA (270mA) 68VA (296mA)
Fuse 1 x T1AL 250V, 20mm long 1 x T1AL 250V, 20mm long 1 x T1AL 250V, 20mm long
Flow Rate min flow 7 L/min, max min flow 7 L/min, max min flow 9.5 L/min, max
flow 9 L/min @ 138 kPa* flow 9 L/min @ 138 kPa* flow 10.5 L/min @ 138 kPa*
Aerosol output rate 0.38 ml/min 0.39 ml/min 0.46 ml/min
Particle Size (MMAD) 3.4µ 3.4µ 3.2µ
Weight 2.9kg (w/o starter pack) 2.7kg 2.7kg
Dimensions 363 x 230 x 118mm 210 x 210 x 185mm 210 x 210 x 185mm
Type Rating Class II BF Continuous Class II BF Continuous Class II BF Continuous
Sound Pressure Level 60 dbA approx. 63 dbA approx. 63 dbA approx.
Intended Use Heavy Heavy Heavy

8
en
Product Suitability
Hospital 333
Clinic 333
Home 377
*1 kPa = 0.01bar or 0.145psi
10. Particle Size Distribution
0.00
10.00
20.00
30.00
40.00
50.00
60.00
70.00
80.00
90.00
100.00
0.101.0010.00 100.00
Cumulative Mass (%)
Aerodynamic Diameter (µm)
Results based on Melbourne Scientific BS EN 13544 - 1 Test Results (Ref AA118) 70% of particles <5µm
AC2000
Econoneb
Turboneb
Mean
Particles <5µm
Plot of Cumulative Size Distribution for Microneb III & Medix Compressors
Cumulative Mass (%)
AC2000
Econoneb
Turboneb
Mean
Particles <5µm
Aerodynamic Diameter (µm)
Plot of Cumulative Size Distribution for Microneb III & Medix Compressor Nebulisers
Results based on Melbourne Scientific BS EN 13544-1 Test Results (Ref AA118) 70% of particles <5µm
Compressor Nebuliser performance is based upon testing that utilises adult ventilatory patterns, these are likely to
be different from those stated for paediatric or infant population.

9
en
11. Guarantee
This guarantee is offered to you as an extra benefit and does not affect your statutory rights.
CCI products are carefully designed, manufactured and inspected. CCI undertake to replace or repair any part found to
be defective in material or workmanship subject to the following terms and conditions:
The compressor/motor, excluding serviceable parts, are guaranteed for three years on condition that:
•the unit is serviced every 12 months for the first three years
•cleaned and maintained in accordance with the instructions
•servicing is undertaken by CCI approved engineers/agents
•only CCI approved parts may be used to service this equipment.
This guarantee does not apply to:
•The disposable medication chamber, driveline, face mask, mouthpiece and filters.
•Defects arising from misuse, negligence, improper maintenance, accident, damage in shipment or unauthorised
modification or service.
•The cost associated with routine 12 monthly servicing.
To register this guarantee please return the card, supplied with this handbook, within 15 days of purchase.
CCI shall not be liable for any third party or consequential loss or damage of whatever nature arising from or in
connection with this equipment. Should it become necessary to make a claim under guarantee, return the unit properly
packed (preferably in the original packaging) to the point of purchase (the manufacturer, shop, distributor or outlet
where the device was purchased).
Please include name and address, details of problem and proof of date of purchase (please retain original invoice).
Carriage costs to be paid by the customer.

10
en
12. EMC (Electromagnetic Compatibility) Declaration
With the increased number of electronic devices such as PC’s and mobiles, medical devices in use may be susceptible
to electromagnetic interference from these devices. Which may result in incorrect operation of the medical device and
create a potentially unsafe situation. Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC with the aim to prevent unsafe product situations, the EN 60601-1-2
standard has been implemented. This standard defines the levels of immunity to electromagnetic interferences as well
as maximum levels of electromagnetic emissions for medical devices.
Medical devices manufactured by Clement Clarke International Ltd. conform to this EN60601-1-2 standard for both
immunity and emissions.
Nevertheless, special precautions need to be observed:
The use of accessories and cables other than those specified by Clement Clarke International Ltd., with the exception of
cables sold by Clement Clarke International Ltd. as replacement parts for internal components, may result in increased
emission or decreased immunity of the device.
The medical devices should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is
unavoidable, the medical device should be seen to operate normally as it should in this situation.
Summary of EMC tests IEC 60601-1-2 (See additional sheet) No deviations occurred.
Electronic devices should be no closer than 30cm (12inches) to any part of the compressor nebuliser system.
Abnormal performance of the compressor nebuliser could be displayed by:
• changes in motor speed
• changes in airflow
Further guidance regarding the EMC environment (in accordance with EN60601-1-2) in which the device should be used
is available at https://www.haag-streit.com/clement-clarke/products/nebulisation/
IMPORTANT
The wires of the mains lead wire are coloured in accordance with the following code:
Blue - Neutral
Brown - Live
As these colours may not correspond to the coloured markings sometimes used to identify the terminals in a plug,
proceed as follows:
Connect the Blue wire to the terminal marked N or coloured black.
Connect the Brown wire to the terminal marked L or coloured red.
NOTE
Neither wire should be connected to the earth pin marked E or coloured green or yellow and green.

11
en
13. Disposal Instruction
All compressor nebulisers, should NOT be disposed of with household waste as they are not biodegradable in landfill
sites. They should NOT be incinerated.
For safe disposal, take to:
The local (council/authority) environmental waste site, (in accordance with the European Environmental directive).
For details contact your local authority/recycling centre.
14. Spares and Accessories
Fuse pack (2 x T1AL 250V) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3605534
Year Pack
Adult year pack . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . L3605122
Contents: 4 x medication chambers, 4 x drivelines, 4 x adult face masks, 1 x mouthpiece,
4 x inlet filters and 1 x outlet filter
Child year pack . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . L3605123
Contents: as above but with child size face masks.
AC2000 only
Shoulder bag . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3607691
Adult year pack (2012) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . L3605125
Contents: as above but with no outlet filter
Child year pack (2012) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . L3605124
Contents: as above but with child size face masks and no outlet filter.
Please note: All our masks are made from a strong plastic with anti-microbial and low static properties and are
phthalate free.


13
fr
Table des Matières
1. Identification du Produit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 14
2. Symboles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 14
3. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 14
3.1 Stockage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 14
4. Kit de Démarrage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 15
5. Conseils d’entretien du nébuliseur . . . . . . . . . . . . . . . . . . . . . . . . . . Page 15
6. Mode d’emploi . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 15
6.1 Remplissage du nébuliseur . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 16
6.2 Administration du médicament . . . . . . . . . . . . . . . . . . . . . . . . Page 16
6.3 Nettoyage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 17
6.4 Changer les Accessoires . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 17
6.5
Remplacer les Filtres
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 17
6.6 Remplacez le fusible . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 18
6.7 Entretien . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 18
7. Consignes de sécurité . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 18
8. Dépannage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 19
9. Spécifications techniques . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 19
10. Distribution granulométrique . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 20
11. Garantie . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 21
12. Déclaration CEM (compatibilité électro-magnétique) . . . . . . . . . . Page 22
13. Fin de vie de votre produit - Recyclage . . . . . . . . . . . . . . . . . . . . . . Page 23
14. Pièces et accessoires . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Page 23
14
fr
1. Identification du Produit
1. Filtre de sortie 5. Off
2. Filtre d’entrée 6. Fusible (T1AL 250V)
3. Interrupteur 7. Cordon d’alimentation
4. On
2. Symboles
Consulter le manuel d’utilisation Fusible (T1AL 250V)
Attention Équipement Classe II
Ne pas mettre la prise au
contact de l’eau Équipement de type BF
Filtre d’entrée Ne pas jeter avec les ordures ménagères
Filtre de sortie
3. Introduction
Ces dispositifs de nébulisation sont conçus pour délivrer le médicament liquide prescrit par votre médecin sous la
forme d’un fin brouillard afin de traiter votre état respiratoire.
Utiliser selon les instructions données par un médecin.
Veuillez lire les instructions attentivement pour obtenir le bénéfice maximum du système
de nébulisation.
Les systèmes de nébulisation à compresseur sont approuvés pour les conditions environnementales /
ambiantes suivantes concernant leur transport, leur utilisation et leur stockage :
3.1Stockage
La machine doit être stockée dans une zone sans fumée ni poussière.
Recommandée : -25˚C - +70˚C
RH : 15% - 90%
Pression : 700hPa - 1060hPa
AVERTISSEMENT : Ne pas utiliser de systèmes de nébulisation à compresseur à proximité d’équipements
chirurgicaux à haute fréquence actifs ou d’équipements électromagnétiques.

15
fr
4. Kits de Démarrage fournis avec l’AC2000 et l’Econoneb qui contiennent:
1 nébuliseur 1 tubulure
1 filtre d’entrée 1 clé à filtre
1 embout buccal 1 masque adulte
1 masque enfant
Le Turboneb 2 n’est pas fourni avec un kit de démarrage.
Le Turboneb 2 fournit le plus haut débit afin de nébuliser les antibiotiques visqueux et a été conçu pour fonctionner sur
secteur. Deux versions sont disponibles, UK et EURO, (soit la prise), 220-240V ~ 50Hz pour les deux.
Ce compresseur offre à l’utilisateur la flexibilité de choisir les accessoires les plus appropriées pour les applications les
plus exigeantes.
Remarque :
•Tous les nébuliseurs à compresseur MEDIX sont conçus pour une utilisation répétitive.
Veuillez vous référer à l’étiquette d’information sur la machine pour vous assurer d’avoir la bonne tension pour
votre alimentation électrique.
• Les pièces énumérées ci-dessus sont nécessaires au bon fonctionnement de ces nébuliseurs à compresseur et
doivent être conformes à la norme EN 13544-1.
5. Conseils d’entretien du nébuliseur
Mise en garde
1. Ne pas immerger l’appareil dans l’eau.
2. Utiliser uniquement selon les prescriptions du médecin.
3. Ne jamais utiliser plus souvent que prescrit.
4. Si la thérapie n’a aucun effet, consulter un médecin.
6. Mode d’emploi (Branchement alimentation)
Température de fonctionnement :
Recommandée : +5˚C - +40˚C
RH : 15% - 90%
Pression : 700hPa - 1060hPa
1. Retirer précautionneusement le matériel de la boîte.
2. Connectez le cordon d’alimentation à une prise de courant.
3. Connectez le tuyau à la fiche du filtre de sortie.
4. Connectez le nébuliseur, assorti d’un masque ou de l’embout buccal, au tuyau.
5. Appuyer sur l’interrupteur principal (I). L’interrupteur va s’allumer. Vérifier le débit d’air avant chaque utilisation.
6. L’équipement peut maintenant être utilisé.
16
fr
6.1Remplissage du nébuliseur
Pour un patient unique
1. Connectez une extrémité de la tubulure au filtre de sortie de votre compresseur.
2. Branchez l’autre extrémité au bas du nébuliseur.
3. Retirez le couvercle du nébuliseur et versez-y le
médicament (max. 10 ml.).
4. Revissez le couvercle sur le nébuliseur.
5. Adaptez le masque ou l’embout buccal sur le couvercle du nébuliseur.
Tous les accessoires doivent être conformes EN 13544-1
6.2Administration du médicament
Allumez le compresseur. Asseyez-vous en position détendue et verticale, placez l’embout dans votre bouche (ou un
masque sur le nez et la bouche) et commencez à respirer lentement et profondément. N’essayez pas de respirer
rapidement. Si vous avez des problèmes prenez conseil auprès de votre médecin ou de votre hôpital.
Éteignez le compresseur quand la brumisation a cessé. Débranchez le cordon d’alimentation.
This manual suits for next models
2
Table of contents
Languages:
Other Clement Clarke International Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Olympus
Olympus ORBEYE OME-V200 instructions
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Shenzhen Mindray Bio-Medical Electronics Co., Ltd. DC-N2 Operator's manual

Vathin
Vathin DVM-A1 user manual

Nasco
Nasco Life/form LF00890U instruction manual

MES
MES SQA-V Gold user guide
dynarex
dynarex 10520 manual