Contec ECG 100G User manual
Other Contec Medical Equipment manuals

Contec
Contec CMS50D User manual

Contec
Contec CMS-50f User manual

Contec
Contec CMS50QB Instruction Manual
Contec
Contec CMS50EW User manual
Contec
Contec CMS-50D Plus User manual

Contec
Contec CMS-50D Plus User manual

Contec
Contec CMS50DL User manual

Contec
Contec CMS50DL1 User manual

Contec
Contec PM-60A User manual
Contec
Contec CMS8000 User manual

Contec
Contec CMS-50E User manual

Contec
Contec ECG1200G User manual
Contec
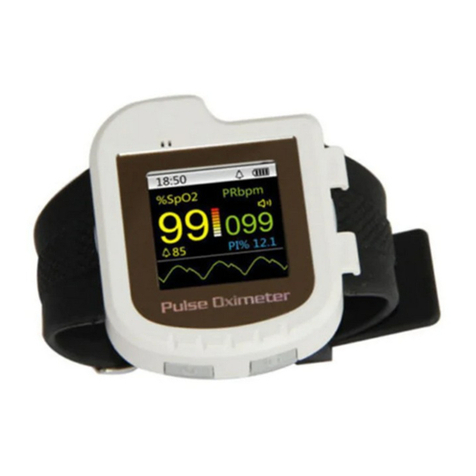
Contec CMS 50IW User manual

Contec
Contec CMS50DL User manual

Contec
Contec CMS50DA User manual

Contec
Contec KT88-3200 User manual
Contec
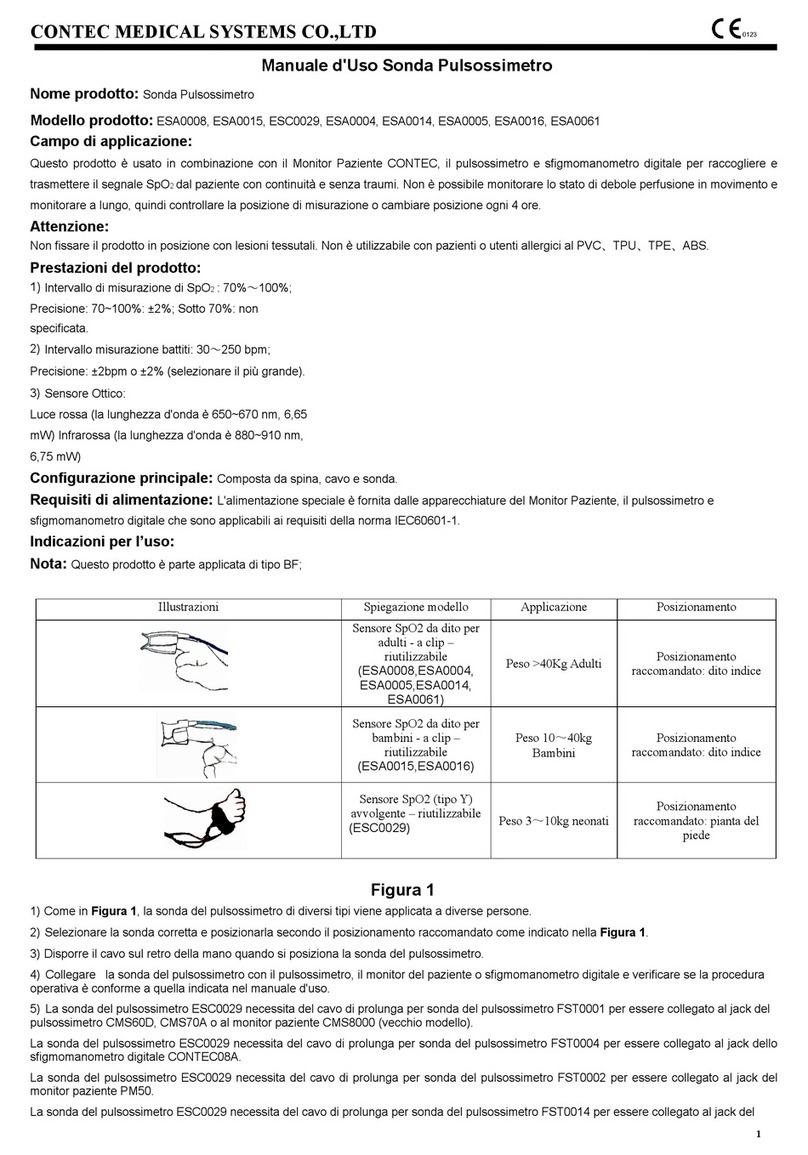
Contec ESA0008 User manual

Contec
Contec CMS-50f User manual

Contec
Contec CMS50D User manual
Contec
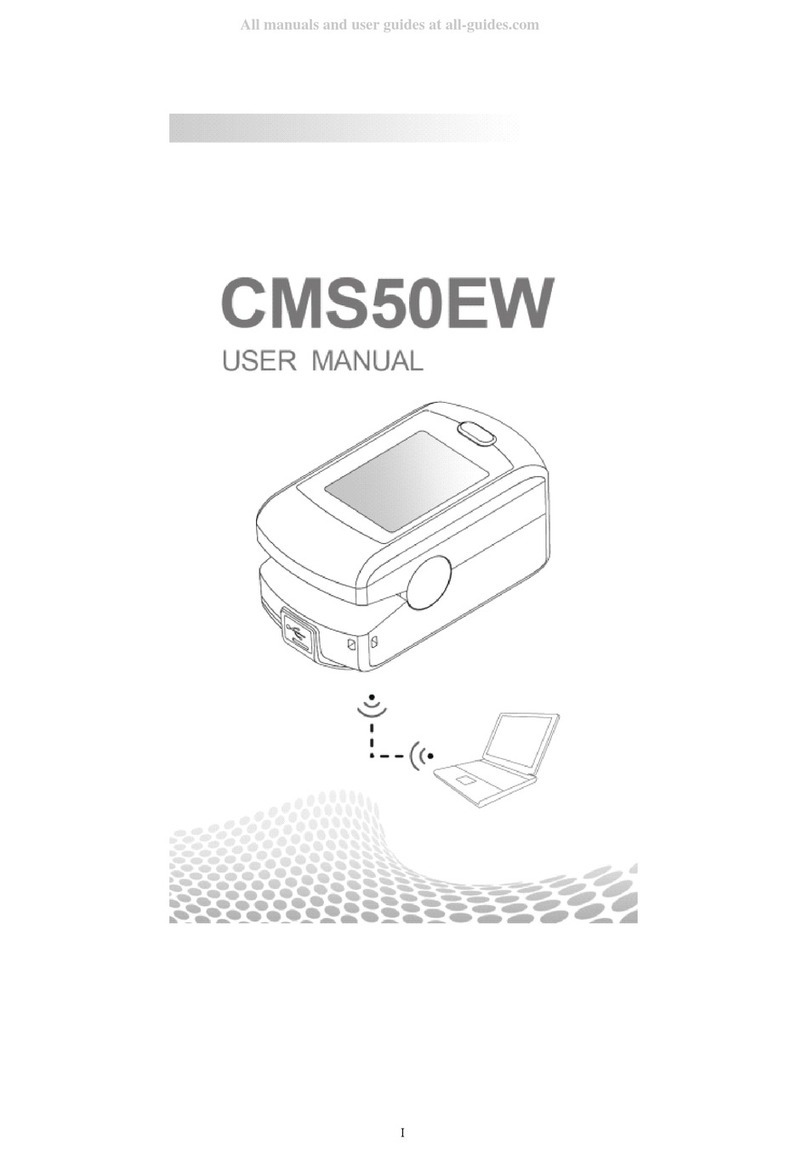
Contec CMS50EW User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual