Contec CMS-50D Plus User manual
Other Contec Medical Equipment manuals
Contec
Contec CMS50EW User manual

Contec
Contec CMS50DL User manual
Contec
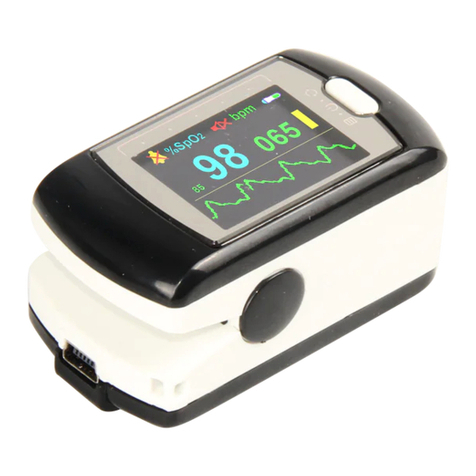
Contec CMS50EW User manual
Contec
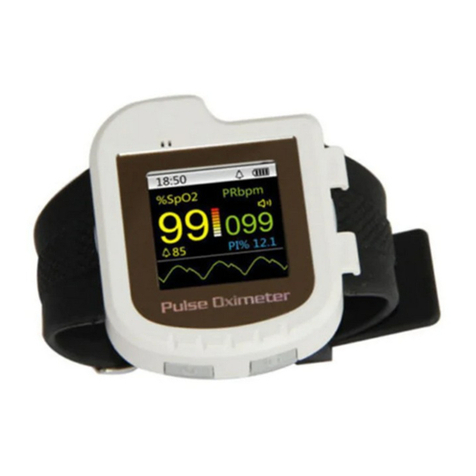
Contec CMS 50IW User manual

Contec
Contec CMS-50f User manual

Contec
Contec CMS-50D Plus User manual
Contec
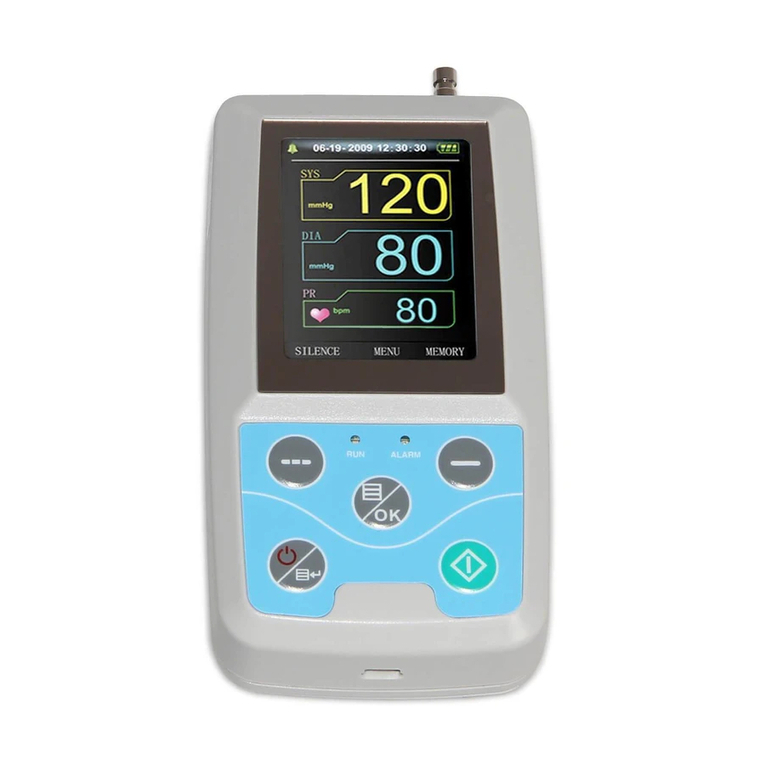
Contec ABPM 50 Reference guide

Contec
Contec CMS-50f User manual

Contec
Contec CMS50DL1 User manual

Contec
Contec MDD93EEC Instruction Manual
Contec
Contec ESA0008 User manual

Contec
Contec CMS50D User manual

Contec
Contec CMS50D User manual

Contec
Contec CMS50DL User manual

Contec
Contec CMS50M User manual

Contec
Contec CMS60C User manual
Contec
Contec CMS60C User manual

Contec
Contec KT88-3200 User manual

Contec
Contec CMS800G User manual
Contec
Contec CMS60C User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual

























