Cristofoli Mini-incubator User manual

01
CRISTÓFOLI
M I N I - I N C U B A D O R A
M A N U A L D E I N S T R U Ç Õ E S
Introduction .......................................................................................................................
Legend of Symbols ...........................................................................................................
Important Safety Information ............................................................................................
Installation Instructions .......................................................................................................
Safety Devices ..................................................................................................................
Mini-incubador Features ...................................................................................................
How to Use the Cristófoli Mini-incubador ...........................................................................
Requirements to be Observed for the ProcessIncubation ................................................
Possible Incubation Failures ...............................................................................................
Preventive Maintenance ...................................................................................................
Troubleshooting .................................................................................................................
Quality Control ..................................................................................................................
How to Identify your Mini-Incubator ...................................................................................
Technical Data .................................................................................................................
Warranty Certificate ..........................................................................................................
How to Proceed When Service is Needed ........................................................................
Guidance for the Final Disposal of the Equipment ...........................................................
Links of Interest ..................................................................................................................
Bibliographical References ...............................................................................................
CONTENTS
01
01
01
02
02
02
02
02
03
03
03
03
03
03
04
04
04
04
04
04
PLEASE, READ ALL THE INSTRUCTIONS IN THIS MANUAL BEFORE USING YOUR CRISTÓFOLI
MINI-INCUBATOR. INCORRECT USE MAY RESULT IN INCUBATION FAILURE, INCORRECT
INTERPRETATION OF THE RESULTS AND/OR ACCIDENTS !
INTRODUCTION
LEGEND OF SYMBOLS
IMPORTANT SAFETY INFORMATION
The intended use of this equipment is to perform the incubation of the self-contained
biological indicators used to monitor the sterilization cycles in steam autoclaves. It is essential
that the operator reads attentively all the instructions before using the m to make ini-incubator
sure they are correctly understood.
WARNING! Always make sure you unplug the equipment to perform any kind of
maintenance or everyday cleaning.
We recommend reading this manual until it is fully understood. Keep it at hand and use it as
a constant reference source;
Do not allow patients or especially children to get close to the device;
Use only the self-contained biological indicator described in this manual;
Never touch the incubation area (Fig. 2, page 2) during or right after using the mini-
incubator. Improper use may cause burns. Cristófoli is not responsible for incorrect
procedures that may cause accidents;
Make sure the mini-incubator’s lid is properly closed before using it. Not following this
procedure will prevent its correct functioning. See “How to Use the Cristófoli Mini-
incubator” (Page 2).
WARNING! Never put any kind of object inside the mini-incubator different than a self-
contained biological indicator or make any use other than the one described in this
manual.
SAFETY STEPS TO PROPERLY OPERATE YOUR CRISTÓFOLI MINI-INCUBATOR
Manufacturer
Cristófoli Equipamentos de Biossegurança Ltda.
Rod. BR 158, nº127 - Campo Mourão - PR - Brasil
CEP 87309-650
CNPJ 01.177.248/0001-95 - Inscr. Est. 90104860-65
www.cristofoli.com - e-mail: [email protected]
Responsible Technician
Eduardo Luiz Soppa
CREA-PR: 109201/D
Manual Mini-incubadora Ingl. Rev.6 2018
LOT
SN
~
This Side Up
Maximum Pile
Caution, consult
accompanying documents
Manufacturer
Alternate Current
Equipment
Class II
Autoclavable
Date of
Manufacture
Caution!
Hot Surface
Boas Práticas
de Fabricação
P
BF
5
Cristófoli, Brazilian company importer and manufacturer of health
products certified by ISO 13485 - Medical Devices - Quality
Management System - Requirements for Regulatory Purposes,
and accordance to the requirements of *BPF - Boas Práticas
de Fabricação - ANVISA.
CRISTÓFOLI’S QUALITY AND ENVIRONMENTAL POLICY
Develop innovative solutions to protect life and promote health.
CRISTÓFOLI’S MISSION
Cristófoli Mini-incubador
13485
D
E
S
G
A
E
M
S
E
T
T
Ã
S
I
O
S
Cristófoli Equipamentos de Biossegurança Ltda., established at Rodovia BR-158, nº 127,
Jardim Curitiba in Campo Mourão, Paraná, Brasil, manufactures biosafety equipment to
assist the health field having as policy: “Develop innovative solutions for the health field by
using agile, robust and objective processes to better serve its clients. Fulfill the requirements
for regulatory purposes of the applicable standards, promote the continuous improvement
of its quality and environmental systems, prevent pollution, reduce its environmental impacts
and continuous training of its employees, achieving this way, a sustainable profitability and
the maximization of the company’s value”. Rev. 2.
Thanks for choosing us. You, our clients, are the reason of Cristófoli's commitment.
We put together this manual to guide you as best as possible, in the use and maintenance
of your Cristófoli Mini-incubador.
We would like to thank all our customers and partners for helping us to continually improve
and innovate our products and services.
Special thanks to Liliana J. P. Donatelli, Cristófoli’s Biosafety Consultant who provides us a
valuable assistance in the coordination of the Cristófoli's Biosafety Project, complementary
products research, our employees, representatives and technicians trainings, and as a
lecturer of Biosafety Courses for professionals, academics and assistants.
For any complaints about our products, please get in touch with our CSD - Customer
Service Department, through the address below.
“Cristófoli. Valuing life!”
This equipment was developed to assist you in the important procedure of incubating the
self-contained biological indicators used to monitor steam sterilization autoclave cycles. We
dedicated ourselves intensively to guarantee you the highest quality and safety. We hope to
obtain this way, the highest level of satisfaction from our clients.
The purpose of this manual is to familiarize you with the features and proper operation of
your Cristófoli Mini-incubator so you know how to take good care of it, obtain the best results
in the incubation process, as well as increase the equipment lifespan.
We recommend extra attention to this manual, the incubation process, although simple,
requires familiarization with the procedure.
All the data on biosafety in this manual were taken, fully or partially, from publications of
renowned biosafety professors based on the Brazilian Legislation or on International
Standards to offer updated information about the monitoring process.
It is important to know some aspects that can void this warranty as a result of negligence,
improper use, non-authorized repairs, etc.
You will find the Warranty Certificate on page 4.
*BPF - Boas Práticas de Fabricação: Brazilian standard similar to the GMP - Good Manufacturing Practices (FDA / US).
Fragile
Handle with Care
Serial Number
Batch Number
Keep Away from
Sunlight
Recyclable
Keep Dry
13485
D
E
S
G
A
E
M
S
E
T
T
Ã
S
I
O
S
ISO 13485
135 ºC
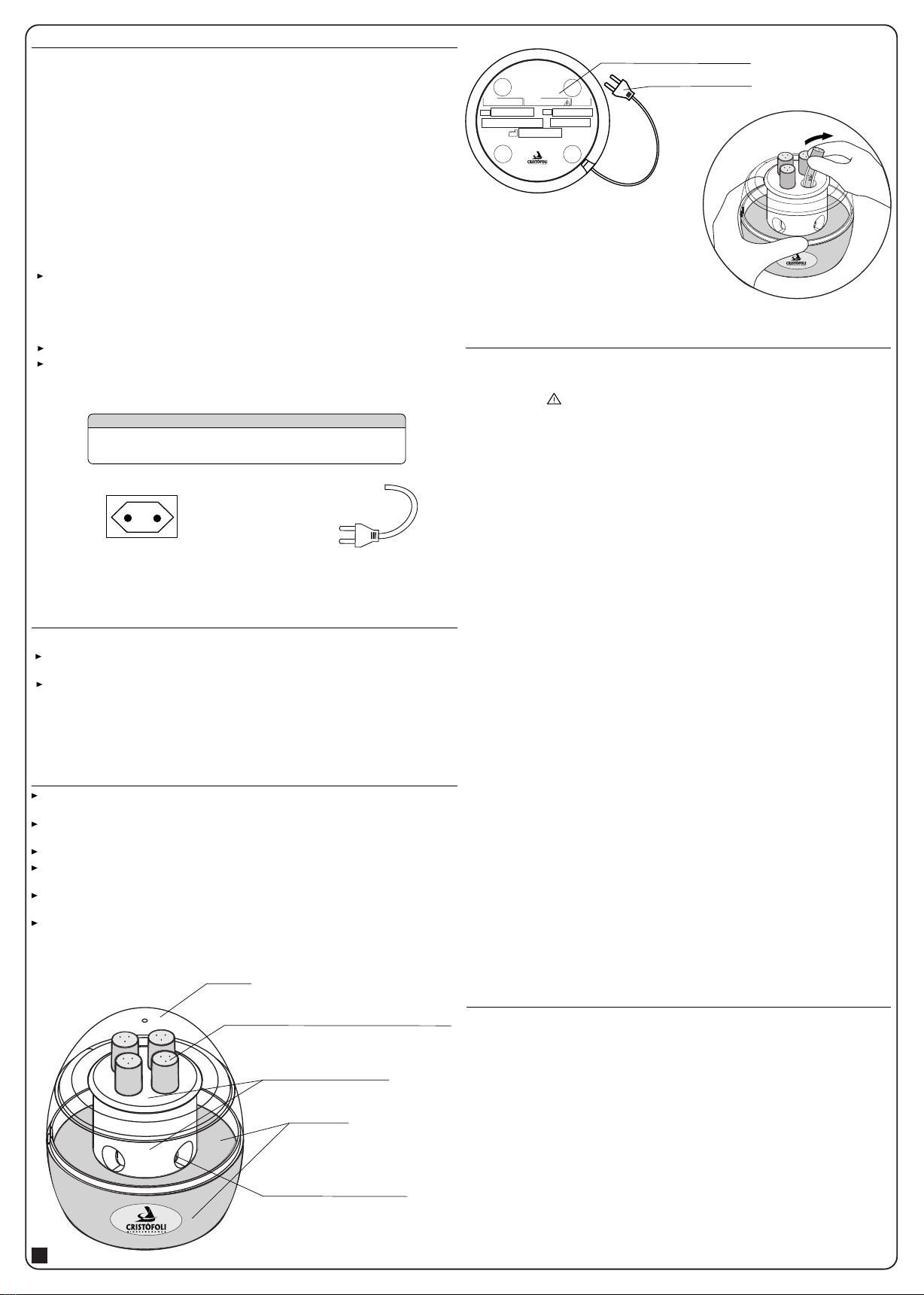
PHYSICAL INSTALLATION
Install the mini-incubator on a flat, leveled and firm surface, at an adequate height for the
operator to handle it. Leave enough room close to the mini-incubator for the proper handling
of the biological indicators to be incubated. The place where the mini-incubator will be
installed must be ventilated, clean and apart from the place where the patients are treated.
The ideal installation should be done in a separate sterilization and/or material processing
room.
Important! Install your mini-incubator where it can be easily unplugged. This is the proper
way to turn the equipment off completely.
ELECTRICAL INSTALLATION
The voltage of the Cristófoli Mini-incubator is from 90V to 253V (automatic voltage
selection), as informed in the identification label located under the device (Fig. 3). Make
sure the wiring voltage where it will be installed is in accordance with the values marked in
the field Voltage of the Identification Label. ATTENTION! Non compliance with this
requirement may damage your mini-incubator. Cristófoli is not responsible for
damages caused by inappropriate installations and/or voltage.
Use a common outlet to plug it in (two pins, Figs. 1 and 1A).
Never use extensions, voltage transformers or any kind of adapters.
Your Cristófoli Mini-incubator has the following safety devices:
The electrical installation must follow the data from the table below:
SAFETY DEVICES
LID - It closes the incubation area during operation, helping to maintain the temperature
stable and protect the operator from accidental burns (Fig. 2).
- PRINTED FUSE Built into the electronic circuit, its purpose is to protect the building electrical
wiring against peaks of energy.
MAIN UNIT - It is made with injected ABS plastic, white on the lower half and transparent on
the top half (Fig. 2).
LID - Its function is to close the incubation area during operation to maintain the
temperature stable (Fig. 2).
POWER CABLE - Used to connect the equipment to the electric outlet (Fig. 3).
INCUBATION AREA - Screwed to main unit, it is where the indicators are inserted for
incubation. It has a capacity for 4 biological indicators (Fig. 2).
LED - It indicates when the mini-incubator is on, it also illuminates the incubation area,
making the visualization of the results easy and clear (Fig. 2).
IDENTIFICATION LABEL - Affixed under the device its function is to identify the mini-,
incubator technical data ( ). Fig. 3
MINI-INCUBATOR FEATURES
1
2
HOW TO USE THE CRISTÓFOLI MINI-INCUBATOR
ATTENTION! Before starting the incubation procedures, the operator must be using latex
gloves. Handle the biological indicators carefully. The mini-incubator must be installed in a
proper and exclusive sterilization and/or materials processing room.
The symbol 14 appears in the identification label of the mini-incubator, that means the
selected item requires special attention and that the user/operator must observe its
reference in the instruction manual provided with the equipment regarding the potential
hazard the device may offer and any actions to be taken should an adverse situation occur.
Cristófoli Equipamentos de Biossegurança Ltda., is not responsible for any failures and/or
accidents caused by the non-observance of this warning.
ATTENTION! Use only self-contained biological indicators for steam sterilization with the
mini-incubator. Non-observance of this recommendation may cause incubation failure,
poor interpretation of the results and/or damage to the device, as well as loss of warranty.
“Correct functioning of sterilization cycles should be verified for each sterilizer by the
periodic use (at least weekly) of Bis”. (Centers for Disease Control and Prevention.
Guidelines for Infection Control in Dental Health-Care Settings - 2003. MMWR;52 (No. RR-
17): page 24). Each institution must also, according to the Brazilian Health Ministry,
establish a monitoring routine of its autoclaves, with the objective of detecting faults in
their equipment and /or process.
In hospitals, monitoring must be done on a daily basis (AORN, 1994). A biological
indicator must be used for every sterilizer load that contains an implantable device. The
load can only be released after the results of incubation are known.
Use only biological indicators for steam (56ºC)
1 - Plug the mini-incubator into the outlet. The white LED will light indicating the device is
on;
Note: The incubation temperature is 56°C. Wait for the temperature adjustment which will
take from 40 to 60 minutes;
2 - After being sterilized according to its manufacturer instructions, remove the indicator
from the autoclave, wait 15 minutes, activate it and insert it into the incubation area,
activate also another indicator from the same batch that was not autoclaved to serve as
a control;
Note: To activate the biological indicator, hold the mini-incubator firmly and insert it half
way in any of the available incubation orifices (Fig. 4), press it carefully in any direction
until you feel that the internal glass ampoule has broken, bring it back to its due position
and slide it into the incubation area.
3 - Wait for the correct incubation time according to the instructions of your biological
indicator manufacturer (24 or 48 hours);
Note: It is important that during the incubation period, the lid is always closed to keep the
temperature steady, open it only when necessary (insert or remove the biological
indicators).
4 - After the time needed for the incubation has elapsed, remove the biological indicator
from the incubation area and check the results.
It is recommended to examine the biological indicators at regular intervals to verify the
color change (example, 12, 16, 20, 24 and 48 hours). Bacterial growth is indicated by the
color change of the biological indicator according to the manufacturer instructions.
Note: To cancel the incubation process, simply unplug it from the electric outlet.
We suggest that the operator standardize the incubation process and prepare a written
routine to avoid leaving any requirement behind.
INDICATORS STORAGE
The storage of the biological indicators must follow the instructions from the manufacturer,
specially the temperature exposure limits. The cabinets must be easy to clean, located in a
dry room with fresh air, free from odors, disinfectants and/or sterilants.
BIOLOGICAL INDICATORS SHELF LIFE
The shelf life / expiration date is generally printed on the label and/or box of the biological
indicators, it is the date after which they cannot be used. We recommend the use of the
“SporTest” biological indicators which have an 18-month shelf life.
INCUBATION PROCESS MONITORING
Monitoring is nothing more than controlling the incubation with self-contained control
indicators. The control indicators (indicators that have not been sterilized), have the important
function of evaluating the viability of the test indicators, as well as the capacity of the
incubator in providing the ideal conditions to incubate them. In case the result from the
control is not positive, it is possible that there is some problem with that batch of indicators,
with their storage, or even with the incubator. Check if a power outage occurred during the
incubation process. If that is the case, perform the whole test again. That means a new cycle
must be performed in the autoclave, containing a test indicator and the incubation process
REQUIREMENTS TO BE OBSERVED FOR THE INCUBATION PROCESS
Fig.1A
Fig.1
Bipolar outlet and plug
(two pins) 10 A.
Fig.2
LID
INCUBATION AREA
BIOLOGICAL INDICATORS (illustrative)
MAIN UNIT
LED (located inside the
incubation area)
POWER CABLE
Fig.4
IDENTIFICATION LABEL
Fig.3
2
3
02
MODEL AMPERAGE VOLTAGE
Cristófoli
Mini-incubator 0,07 90V - 253V AC
automatic
voltage selection
1
The storage/installation must be done in a place protected from the weather action
(indoors) in normal conditions of ambient temperature.
The Cristófoli Mini-incubator can be easily installed. Check if the wiring voltage of your
building is in accordance with the mini-incubator specifications consulting the “Technical
Data” (Page 3) at the time of installation. See “Warranty Certificate” and “How to Proceed
When Service is Needed” (Page 4).
INSTALLATION INSTRUCTIONS
CRISTÓFOLI
EQUIPAMENTOS DE BIOSSEGURANÇA LTDA
ROD BR 158 Nº 127 - CAMPO MOURÃO - PR - BRASIL
CNPJ 01.177.248/0001-95
INSCR. EST. 90.104.860-65
CEP 87303-327
TEL: 55 44 3518-3434
MADE IN BRAZIL
CAPACITY
4 Indicators
Cristófoli Mini-incubator
MODEL
50/60 Hz
S N
90 - 253V~ - 0,07 kW - 0,1A
VOLTAGE
PRODUCT: INCUBATOR FOR BIOLOGICAL INDICATORS
USE INSTRUCTIONS, PRECAUTIONS, CONSERVATION AND STORAGE:
SEE INSTRUCTION MANUAL.
RESPONSIBLE TECHNICIAN
Eduardo Luiz Soppa
CREA-PR: 109201/D
ANVISA REGISTER
EXEMPT
According to Resolution RDC nº 260/02
LOT
FREQUENCY

03
The mini-incubator is not connected to the
electric outlet; Plug the mini-incubator into the electric
outlet;
See “How to Proceed when Service is
Needed” (Page 4);
Printed fuse burned out;
No power; Check if there is a power outage in your
building;
SOLUTIONSPOSSIBLE CAUSES
THE MINI-INCUBATOR DOES NOT TURN ON
Defective circuit board; See “How to Proceed when Service is
Needed” (Page 4);
SOLUTIONSPOSSIBLE CAUSES
A MINI-INCUBADORA LIGA, MAS NÃO AQUECE
SOLUTIONSPOSSIBLE CAUSES
If the problem persists after the verification of all the items listed,
contact your local dealer.
The mini-incubator lid is open; In case the lid of the incubation area is open,
close it properly;
Defective circuit board; See “How to Proceed when Service Is
Needed” (Page 4);
A TEMPERATURA DA MINI-INCUBADORA SOBE EXCESSIVAMENTE
All Cristófoli Mini-incubators are tested and monitored individually, according to the
parameters of the table below.
QUALITY CONTROL
NOTE: Heating Time values are expressed considering the technical data chart regarding the
environment conditions, (temperature / altitude). Incubation time will depend on the
biological indicator used. (check the time needed for your BI by consulting its manufacturer
instructions.
A MINI-INCUBADORA DEMORA PARA ATINGIR TEMPERATURA OU NÃO A MANTÉM
SOLUTIONSPOSSIBLE CAUSES
The metallic identification label is located underneath the equipment. Its purpose is to
identify the technical data.mini-incubator
ATTENTION! - Removing the Identification Label and/or any other stickers from the product will
cause automatic loss of the warranty.
HOW TO IDENTIFY YOUR MINI-INCUBATOR
Fig.5
* In case the altitude and/or temperature of your work place is different from the one
mentioned in this manual, contact Cristófoli by the e-mail: [email protected].
Note: The manufacturer reserves the right to make changes and/or improvements to this
product at any moment without prior notice.
CERTIFICATIONS ..........
CAPACITY .....................................................
WEIGHT.........................................................
CYCLE .........................................................
OVERALL CLEARANCE ..................................
CLEARANCE REQUIRED TO OPEN THE LID ......
EXTERNAL DIMENSIONS (H x D) .....................
LID DIMENSIONS (H x D) ................................
VOLTAGE .....................................................
FREQUENCY .................................................
POWER ........................................................
POWER CONSUMPTION ................................
MAXIMUM TEMPERATURE ..............................
*PROPER WORKING TEMPERATURE ................
*PROPER WORKING ALTITUDE .......................
4 biological indicators
240 g
24 o 48 hours (steam)
15 cm for each side
12 cm
8,7 x 7,6 cm
3,3 x 7 cm
90 - 253V AC single-phase
50/60 Hz
10 Watts
10 Watts hour
60 ºC
15 ºC to 40 ºC
Up to 2.000 m
TECHNICAL DATA CHART CRISTÓFOLI MINI-INCUBATOR
Cristófoli Mini-incubators are manufactured by Cristófoli Biossegurança,
company which Quality Management System is certified by ISO
13485:2003, and in accordance with BPF - Boas Práticas de Fabricação -
ANVISA (Brazilian standard similar to GMP - FDA / US).
TROUBLESHOOTING
WARNING! For any parts replacement, contact your local dealer or the manufacturer. We
listed below some possible problems and solutions you can try on your own.
TECHNICAL DATA
24 or 48 hours
(according to the B. I.)
55 ºC to 60 ºC
Cold or hot Mini-incubator
60 minutes
PREVENTIVE MAINTENANCE
Some preventive procedures are necessary for the best functioning and durability of your
mini-incubator:
Use only self-contained biological indicators, proper for steam sterilization;
incubator the incubation area, use a cotton swab Keep the mini- clean. To clean with
alcohol 70% or peracetic acid at 1% and rub inside the indicators compartments some
times cause d s.. The use of other materials and/or products may amage
The external cleaning must be done daily using a soft cloth and biodegradable neutral
detergent, next, clean it thoroughly with a soft cloth and alcohol 70%or peracetic acid at
1%.
Insufficient exposure time to the proper temperature;
Incorrect handling of the incubator or the biological indicators;
Inappropriate indicators for incubation (not proper for steam, incorrectly stored or overdue
shelf life);
Removal of the lid, causing the incubation temperature to drop beyond the ideal level;
Lack of preventive maintenance;
Power outage;
Defective incubator.
POSSIBLE INCUBATION FAILURES
1
2
3
4
6
7
5
ATTENTION! - Failures during the incubation process are detected during monitoring.
must be repeated with a new control indicator.
AUTOCLAVE STERILIZATION MONITORING
The purpose of the Biological indicators is to monitor autoclave sterilization cycles, they are
considered the most accepted method for monitoring the sterilization process because they
assess it directly by killing known highly resistant microorganisms (e.g., Geobacillus
stearothermophilus for steam and Bacillus subtillis for ethylene oxide), rather than merely
testing the physical and chemical conditions necessary for sterilization. Because spores used
in BIs are more resistant and present in greater numbers than the common microbial
contaminants found on patient-care equipment, an inactivated* BI indicates other potential
pathogens in the load have been killed. The articles must have been previously cleaned
before being sterilized, since the organic matter protects the microorganisms from the action
of the sterilizing agents.
“Correct functioning of sterilization cycles should be verified for each sterilizer by the
periodic use (at least weekly) of Bis”. (Centers for Disease Control and Prevention.
Guidelines for Infection Control in Dental Health-Care Settings - 2003. MMWR;52 (No. RR-
17): page 24). Each institution must also, according to the Brazilian Health Ministry,
establish a monitoring routine of its autoclaves, with the objective of detecting faults in
their equipment and /or process.
In hospitals, monitoring must be done on a daily basis (AORN, 1994). A biological
indicator must be used for every sterilizer load that contains an implantable device. The
load can only be released after the results of incubation are known.
The number of test indicators to be put in each piece of equipment must be
established according to its volume. A control BI, from the same lot as the test indicator
and not processed through the sterilizer, should be incubated with the test BI; the control BI
should yield positive results for bacterial growth and the results from the test BI should be
negative. The incubation process must begin from 15 minutes after the end of the
sterilization, and no later than two hours after it.
( * inactivated = negative).
Heating Time
Incubation Time
Temperature
CRISTÓFOLI
EQUIPAMENTOS DE BIOSSEGURANÇA LTDA
ROD BR 158 Nº 127 - CAMPO MOURÃO - PR - BRASIL
CNPJ 01.177.248/0001-95
INSCR. EST. 90.104.860-65
CEP 87303-327
TEL: 55 44 3518-3434
MADE IN BRAZIL
CAPACITY
4 Indicators
Cristófoli Mini-incubator
MODEL
50/60 Hz
S N
90 - 253V~ - 0,07 kW - 0,1A
VOLTAGE
RODUCT: INCUBATOR FOR BIOLOGICAL INDICATORS
USE INSTRUCTIONS, PRECAUTIONS, CONSERVATION AND STORAGE:
SEE INSTRUCTION MANUAL.
RESPONSIBLE TECHNICIAN
Eduardo Luiz Soppa
CREA-PR: 109201/D
ANVISA REGISTER
EXEMPT
According to Resolution RDC nº 260/02
LOT
FREQUENCY

04
LINKS OF INTEREST
www.anbio.org.br Biosafety National Association
www.anvisa.gov.br ANVISA - Brazilian National Sanitation Surveillance Agency
www.ccih.med.br Site of the book “Hospital Infections”
www.cdc.gov Centers for Disease Control and Prevention (Atlanta-GA-USA).
www.cristofoli.com Cristófoli’s website
www.fob.usp.br Bauru Dentistry College
www.saude.gov.br Brazilian Health Ministry
www.saude.sp.gov.br São Paulo Health Department
APECIH- ASSOCIAÇÃO PAULISTA DE ESTUDOS E CONTROLE DE INFECÇÃO HOSPITALAR.
Esterilização de Artigos em Unidades de Saúde. 1998.
APECIH- ASSOCIAÇÃO PAULISTA DE ESTUDOS E CONTROLE DE INFECÇÃO HOSPITALAR. Limpeza,
Desinfecção de Artigos e Áreas Hospitalares e Antissepsia. 1999.
APECIH- ASSOCIAÇÃO PAULISTA DE ESTUDOS E CONTROLE DE INFECÇÃO HOSPITALAR. Controle
de Infecção na Prática Odontológica. 2000.
APECIH- ASSOCIAÇÃO PAULISTA DE ESTUDOS E CONTROLE DE INFECÇÃO HOSPITALAR.
Esterilização de Artigos em Unidades de Saúde. 2. ed., 2003.
BRASIL. Ministério da Saúde AGENCIA NACIONAL DE VIGILÂNCIA SANITÁRIA - RDC50 -
Regulamento técnico para planejamento, programação, elaboração e avaliação de
projetos físicos para estabelecimentos assistenciais de saúde. Brasília, 2002.
BRASIL. Ministério da Saúde AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA Serviços
Odontológicos: Prevenção e Controle de Riscos Brasília; Ministério da Saúde, Brasília, 2006 a.
156 p.
DONATELLI, L.J.P. Manual de Biossegurança para Odontologia. 2008.
FERNANDES, A.T.; FERNANDES, M. O.; RIBEIRO FILHO, N. Infecção Hospitalar e suas Interfaces
na Área da Saúde. Editora Atheneu, 2000.
FOB. Faculdade de Odontologia de Bauru. Manual de Biossegurança. Universidade de São
Paulo, 2000.
GUANDALINE, S. L.; MELO, N.; SANTOS, E.C.P. Biossegurança em Odontologia. Editora Edelbra,
2ª. ed., 1999.
GUIMARÃES JUNIOR, J. Biossegurança e Controle de Infecção Cruzada em Consultórios
Odontológicos. São Paulo: Livraria Santos, 2001.
ISO 15223 - Medical Devices - Symbols to be Used with Medical Device Labels, Labelling and
Information to Be Supplied. Amendment 1, Agosto 2002.
ISO 15223 - Medical Devices - Symbols to be Used with Medical Device Labels, Labelling and
Information to Be Supplied, Abril 2000.
MINAS GERIAS (ESTADO) Resolução SES Nº.1559. Aprova o Regulamento Técnico que
estabelece condições para a instalação e funcionamento dos Estabelecimentos de
Assistência Odontológica - EAO no Estado de Minas Gerais, 2008.
NBR 12914 - Símbolos gráficos próprios para aplicar em equipamento elétrico utilizado na
prática médica ABNT. 1993.
NBR ISO11138 - Esterilização de produtos para saúde - Indicadores Biológicos - parte 1 –
Requisitos Gerais 6-2004.
NS EN 1041 - Information supplied by the manufacturer of medical devices, Fevereiro 1998.
NS-EN 980 - Graphical Symbols for Use in the Labelling of Medical Devices, Maio de 1996.
SÃO PAULO (ESTADO) Resolução SS 15. Norma Técnica Especial Referente ao Funcionamento
de Estabelecimentos de Assistência Odontológica. 1999.
SÃO PAULO (ESTADO) Resolução SS 374. Norma Técnica sobre Organização do Centro de
Material e Noções de Esterilização. 1995.
TEIXEIRA, P.; VALLE, S. (orgs) Biossegurança - Uma Abordagem Multidisciplinar. Editora Fiocruz,
2002.
BIBLIOGRAPHICAL REFERENCES
GUIDANCE FOR THE FINAL DISPOSAL OF THE EQUIPMENT
The environment is something that belongs to everyone, therefore it is up to each one
of us to make the decisions that will help in its preservation and reduce the damages
resulting from human activities.
All equipment has a useful lifespan, but it is not possible to determine how long, as it
varies according to the intensity and how the equipment is handled.
CRISTÓFOLI EQUIPAMENTOS DE BIOSSEGURANÇA LTDA recommends users of their
products to seek the best destination when disposing the M or its ini-incubator
components, taking into account the materials recycling legislation effective in your
country.
We advise you take your equipment to recycling specialized companies that due to
the continuous and fast paced development of new recycling technologies and
materials reuse, provide the best way of disposing and recycling it properly, contributing
this way to reduce the consumption of non-renewable raw materials.
It is worth reminding you that your Cristófoli Mini-incubator packaging, as indicated on
the box itself, is recyclable.
Other items to be observed for the preservation of our planet:
•Reduce the amount of consumption material;
•Reuse all durable goods for as long as possible;
•Properly dispose the amalgam residues, mercury contaminates the soil;
•Recycle all possible materials at the end of their useful lifespan;
•Perform the correct separation of all waste.
On behalf of users, we thank you for your concern and cooperation.
Before contacting your local dealer, please, have in hands, the model of your
equipment, serial number and the date of manufacture found on the identification label
located underneath the equipment (Fig. 3, page 2 and Fig. 5, page 3) and a description of
the problem. It will be also necessary to have the original invoice from your dealer to confirm
the date of purchase.
Always contact your local dealer. If you have problems contacting your dealer, contact
us by e-mail: [email protected] or through our website: www.cristofoli.com.
HOW TO PROCEED WHEN SERVICE IS NEEDED
CRISTÓFOLI EQUIPAMENTOS DE BIOSSEGURANÇA LTDA., warranties the Cristófoli Mini-
incubators for 01 (one) year against any manufacturing defect from the date of the purchase
receipt (provided it contains the serial and lot numbers of the equipment), of which 03 (three)
months refer to the legal warranty (established by section II, art. 26, CDC, Brasil) and nine (09)
months to the contractual warranty (arranged in art. 50, CDC, Brasil). Visit our website
www.cristofoli.com and register your product online.
Traveling costs (based on the distance traveled in km) and the stay of the authorized
technician for installation, repair or maintenance before or after the warranty period will be
responsibility of the buyer/owner as well as the freight charges for shipping the equipment to
the authorized technical assistance office for repairs or if necessary, to the factory itself.
CRISTÓFOLI EQUIPAMENTOS DE BIOSSEGURANÇA LTDA., is not liable for damages /
accidents caused by improper use, operation or installation of its products, in this case the
equipment will lose its warranty and the repair will be paid by the buyer / owner.
The warranty will be voided in cases of:
- Problems arising from natural causes (such as floods, lightning, etc.).
- Sinister (theft or robbery).
- Damage caused by accidents, such as: drops, failure of the power supply and/or
building wiring, short circuits, fire, etc..
- Improper installation of the equipment and/or plugging it into an incorrect voltage
outlet.
- Removal and/or tempering of the serial number shown in the identification label of the
product.
- Signs of tampering and/or blotted out data on the purchase or service receipt of the
equipment.
- Signs of external violation of the product or broken factory seal.
- Use different from the intended purpose.
- Changes made to the equipment by the client himself/herself.
- Repairs performed by technicians who are not part of the Cristófoli Authorized Service
Network.
- Noncompliance with any measure or caution recommended in the instruction
manual of the product.
- Lack of periodic maintenance of the equipment or neglect regarding any item
presented on the topic “Preventive Maintenance” of this instruction manual.
The printed fuse and the lid of the incubation area are not covered by this warranty.
ATTENTION! In order to validate the contractual warranty of the product it is necessary to send
a copy of the purchase receipt to the following e-mail address: [email protected].
WARRANTY CERTIFICATE
For better serving our clients, we offer our Cristófoli Biosafety Consultancy Service
Liliana Junqueira de P. Donatelli - Biologist - CRB 18469/01-D
Master in Public Health Cristófoli’s Biosafety Consultant FMB - UNESP -
Other Cristofoli Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual

















