Proprietary and Confidential. Printed copies will not be updated.
MNL-00049 [1] Page 2of 19
Table of Contents
1.0 Control Type........................................................................................................ 3
2.0 Indications for Use ............................................................................................. 3
3.0 Delivery and Inspection ..................................................................................... 3
4.0 Site Selection ...................................................................................................... 4
4.1 Electrical Requirements...................................................................................................................4
5.0 Unpacking and Assembly .................................................................................. 4
5.1 Swivel Stand ....................................................................................................................................4
6.0 Lamp Inspection ................................................................................................. 4
6.1 Lamp Specification Guide................................................................................................................4
7.0 Precautions and Warnings ................................................................................ 5
8.0 Operating Specifications ................................................................................... 7
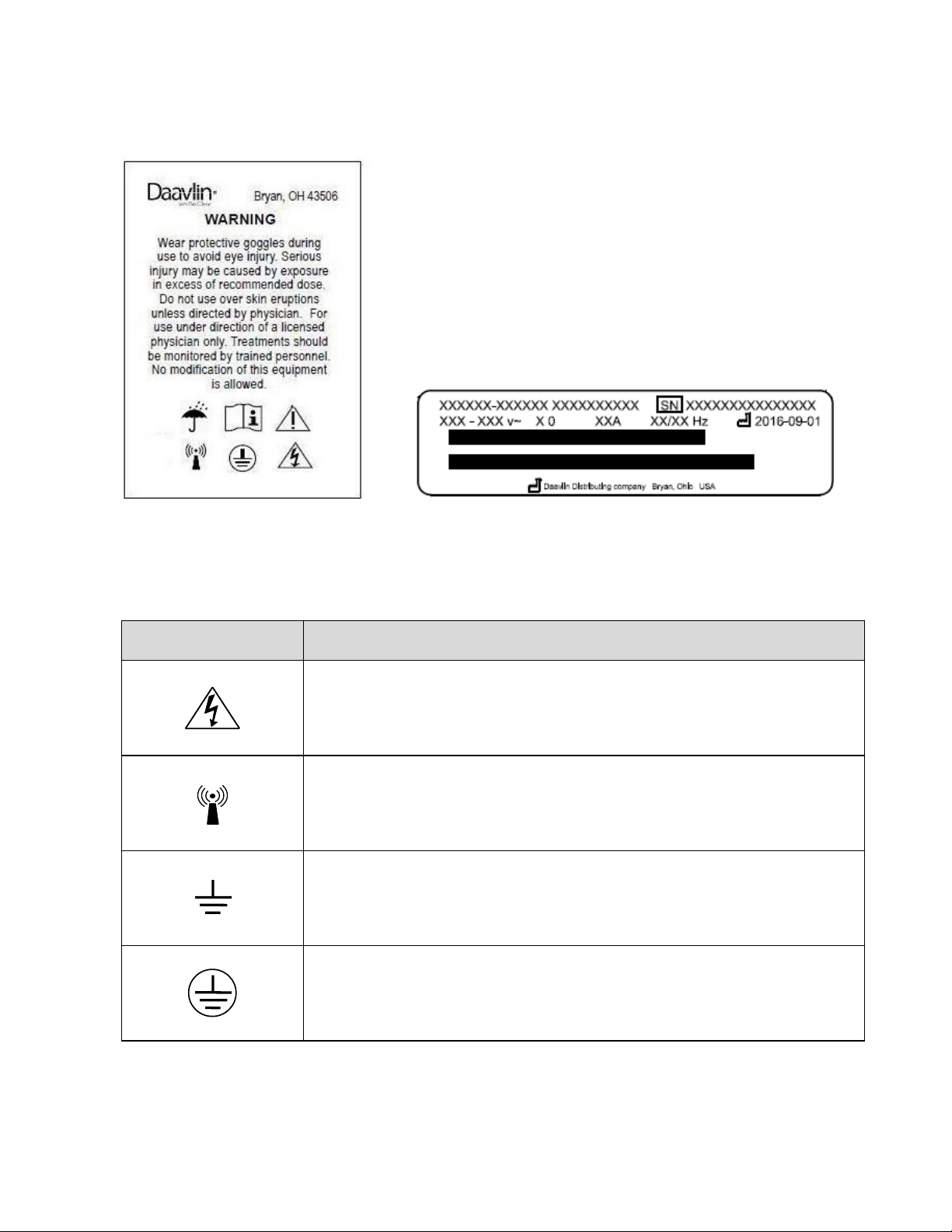
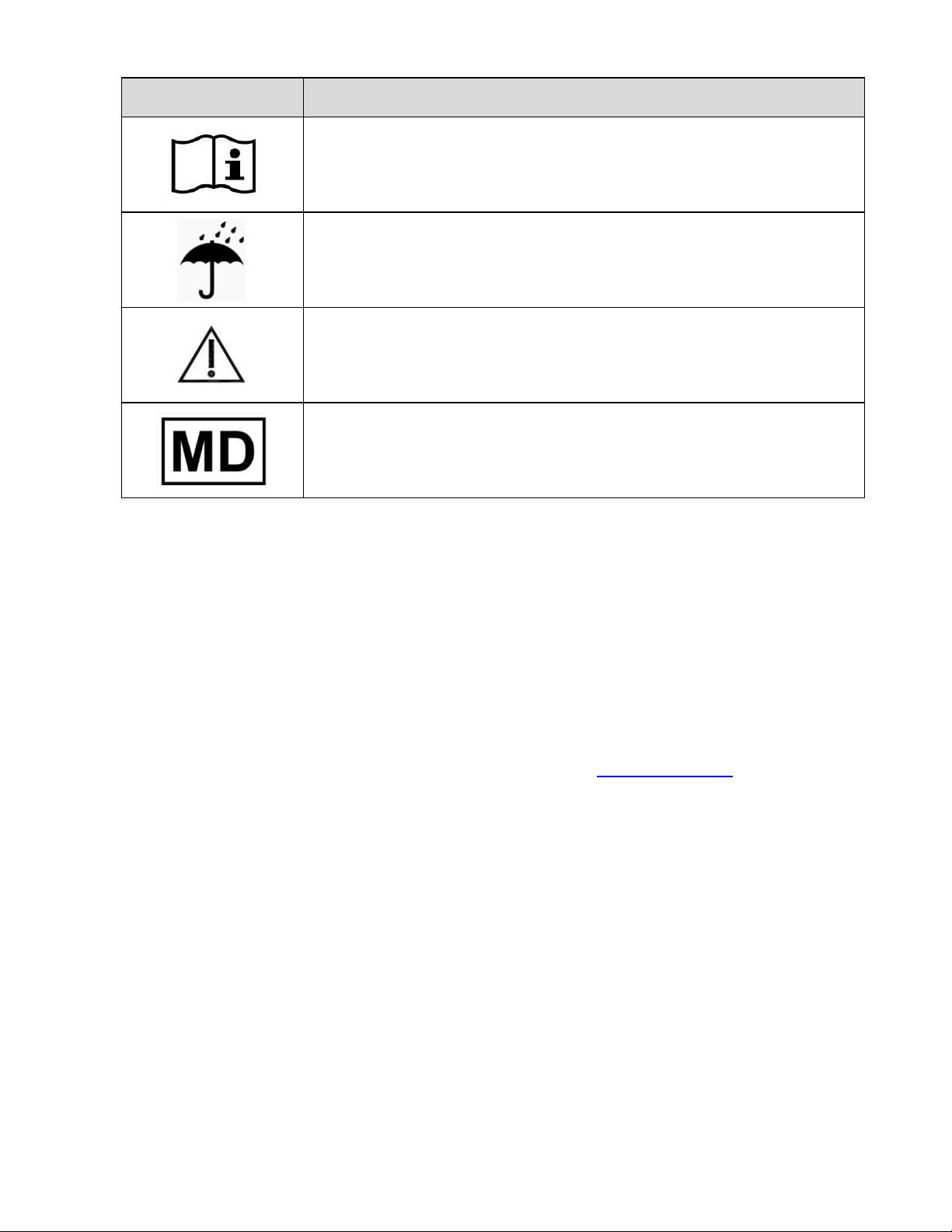
9.0 Labels and Symbols........................................................................................... 7
10.0 General Instructions........................................................................................... 9
10.1 Pre-treatment Preparations..............................................................................................................9
10.2 Unlocking the Device .......................................................................................................................9
10.3 How to Position Yourself................................................................................................................10
11.0 Setting Up a Treatment Time ........................................................................... 10
12.0 Viewing Device Code for Flex Rx .................................................................... 11
13.0 Enable/Disable/Refill FlexRX ........................................................................... 11
13.1 Refill FlexRx...................................................................................................................................12
14.0 Special Notes .................................................................................................... 12
15.0 Care of the Unit................................................................................................. 12
15.1 Recommended Maintenance Schedule.........................................................................................12
15.2 Cleaning/Disinfection .....................................................................................................................13
15.2.1 General Cleaning ................................................................................................. 13
15.2.2 Low-Level Disinfection ........................................................................................ 13
15.2.3 High-Level Disinfection........................................................................................ 13
15.3 Lamp Removal and Replacement..................................................................................................13
15.3.1 Lamp Replacement.............................................................................................. 14
15.3.2 Resetting Lamp Hours......................................................................................... 14
16.0 Environmental Specifications ......................................................................... 14
17.0 Warranty ............................................................................................................ 18
17.1 Limited Warranty Policy .................................................................................................................18
17.2 Warranty Coverage........................................................................................................................18
17.3 Customer Responsibility ................................................................................................................18
17.4 Warranty Service............................................................................................................................18
17.5 Disposal .........................................................................................................................................19
17.6 Other Services ...............................................................................................................................19
17.7 Contact Information........................................................................................................................19