GA 5752 5678 GB 07 3
Table of contents
Table of contents
1 Introduction ..............................................................................................................................................5
1.1 Foreword ....................................................................................................................................................5
1.2 How to use these operating instructions .................................................................................................... 5
1.2.1 Abbreviations ............................................................................................................................... 5
1.2.2 Symbols ....................................................................................................................................... 5
1.2.2.1 Cross-references ....................................................................................................... 5
1.2.2.2 Actions and responses .............................................................................................. 5
1.2.3 Definitions .................................................................................................................................... 6
1.2.3.1 Design of safety notes ............................................................................................... 6
1.2.3.2 Design of other notes ................................................................................................ 6
1.2.4 Symbols used .............................................................................................................................. 6
1.3 Disposal......................................................................................................................................................7
1.3.1 ATMOS products.......................................................................................................................... 7
1.3.2 Packaging .................................................................................................................................... 8
1.4 Overview ....................................................................................................................................................8
1.5 Basic requirements.....................................................................................................................................8
1.5.1 Use in accordance with the intended purpose ............................................................................. 8
1.5.2 Applicable standards.................................................................................................................... 9
1.5.3 Intended purpose ......................................................................................................................... 9
1.5.3.1 Possible applications ............................................................................................... 10
1.5.4 Version LS FLOW flowmeter installation version ....................................................................... 10
1.5.5 Interface description................................................................................................................... 10
1.5.5.1 Flowmeter outlet ...................................................................................................... 10
1.5.5.2 Connection tube ...................................................................................................... 11
1.5.5.3 Tube adapter for Air and O2 .................................................................................... 11
2 Safety notes............................................................................................................................................12
2.1 General safety notes ................................................................................................................................ 12
2.2 Product safety notes.................................................................................................................................12
3 Initial operation.......................................................................................................................................14
3.1 Equipment inspection...............................................................................................................................14
3.2 Mounting accessories...............................................................................................................................14
3.2.1 General ...................................................................................................................................... 14
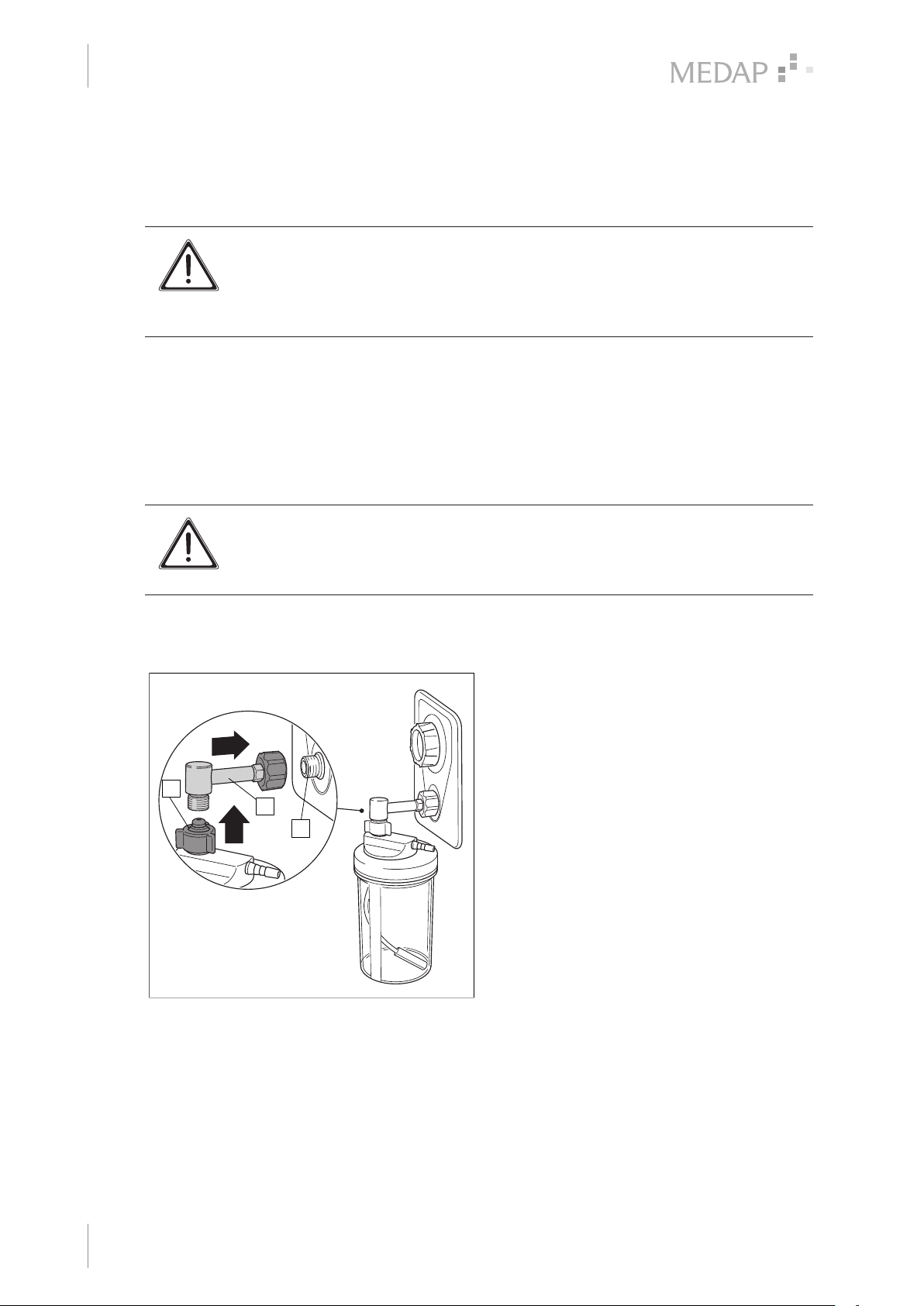
3.2.2 Connection of bubble humidifier (REF 5752 5315).................................................................... 14
3.2.3 Connection of disposable humidifiers from other manufacturers............................................... 14
4 Operation ................................................................................................................................................15
4.1 Function check .........................................................................................................................................15
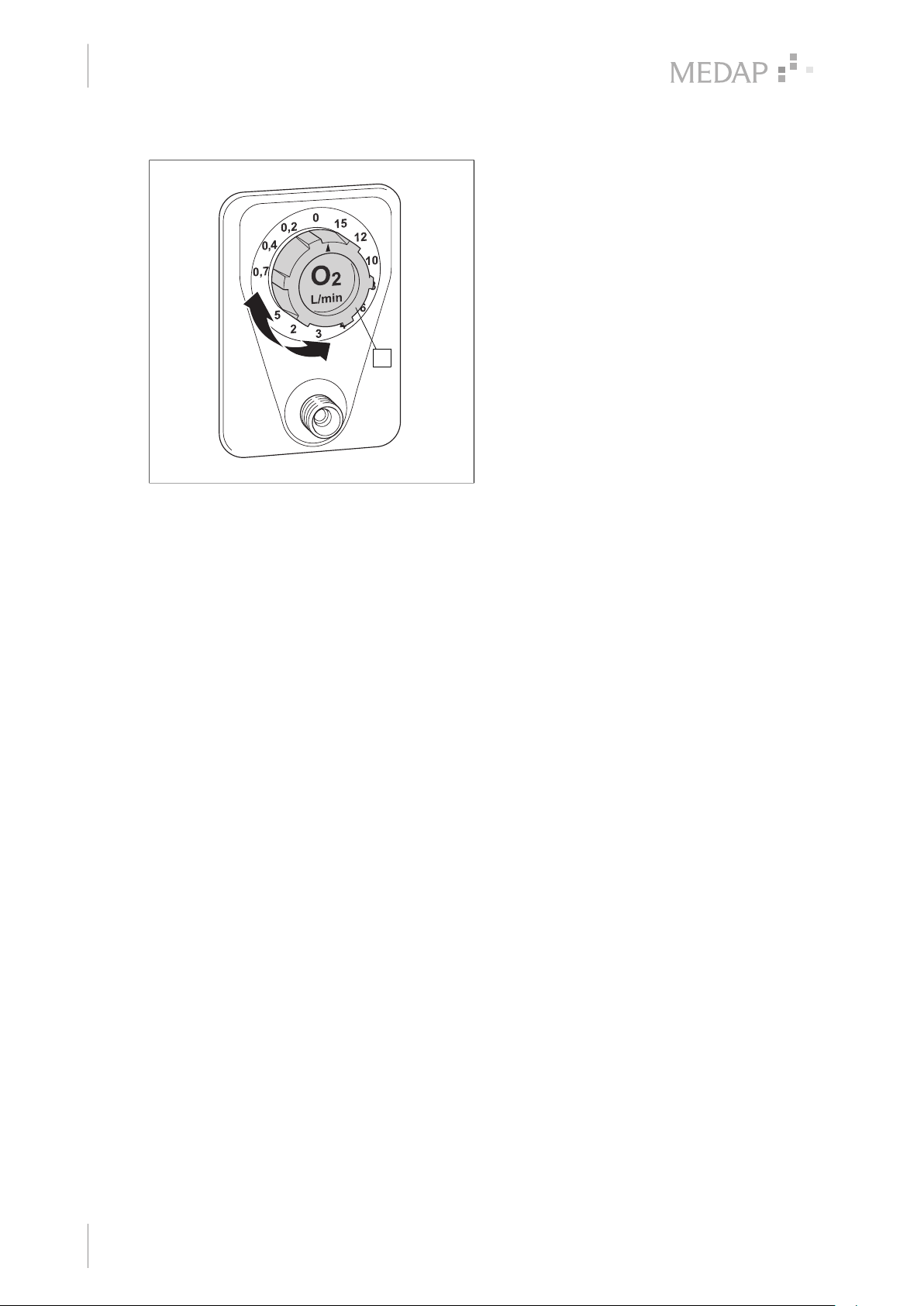
4.2 Setting the flow for treatment ................................................................................................................... 15