Dentsply Sirona Capsule Extruder 2 User manual
Other Dentsply Sirona Dental Equipment manuals

Dentsply Sirona
Dentsply Sirona X-Smart IQ User manual

Dentsply Sirona
Dentsply Sirona DAC Universal User manual

Dentsply Sirona
Dentsply Sirona SmartLite Focus User manual

Dentsply Sirona
Dentsply Sirona SINIUS User manual

Dentsply Sirona
Dentsply Sirona Gutta-Smart Manual

Dentsply Sirona
Dentsply Sirona SiroLaser Blue User manual

Dentsply Sirona
Dentsply Sirona Midwest Stylus Plus User manual

Dentsply Sirona
Dentsply Sirona MIDWEST AUTOMATE User manual

Dentsply Sirona
Dentsply Sirona CEREC Omnicam AC User manual

Dentsply Sirona
Dentsply Sirona inLab Profire User manual

Dentsply Sirona
Dentsply Sirona SiroCam UAF Plus User manual
Dentsply Sirona
Dentsply Sirona X-Smart IQ User manual
Dentsply Sirona
Dentsply Sirona Essix Dual Laminate Plastic Manual

Dentsply Sirona
Dentsply Sirona Eclipse Manual

Dentsply Sirona
Dentsply Sirona Cavitron Select SPS User manual
Dentsply Sirona
Dentsply Sirona Midwest Tradition TB User manual

Dentsply Sirona
Dentsply Sirona X-Smart User manual
Dentsply Sirona
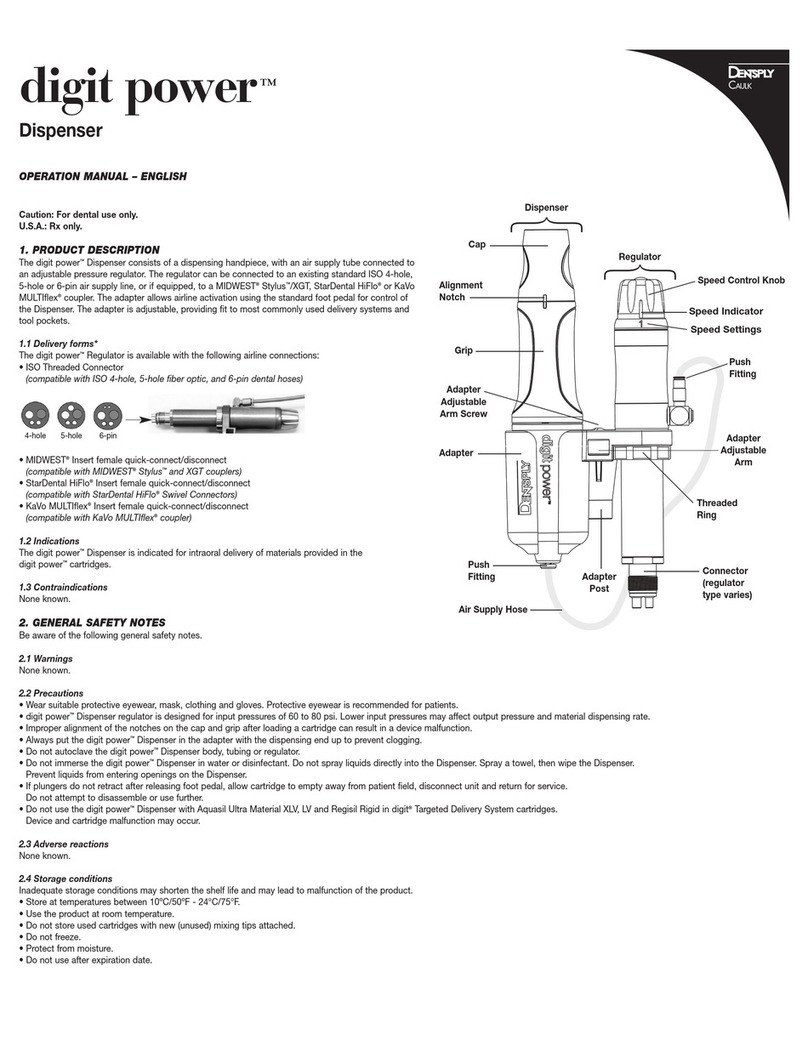
Dentsply Sirona digit Power User manual

Dentsply Sirona
Dentsply Sirona CEREC MC XL User manual

Dentsply Sirona
Dentsply Sirona Midwest Stylus Plus User manual
Popular Dental Equipment manuals by other brands

Vatech
Vatech EzRay Air VEX-P300 user manual

KaVo
KaVo GENTLEpower LUX Contra-angle 25 LP Technician's Instructions

DENTSPLY
DENTSPLY SmartLite Focus Instructions for use

LM
LM ProPower CombiLED quick guide

Owandy Radiology
Owandy Radiology RX-AC user manual

mectron
mectron Piezosurgery Cleaning and sterilization manual