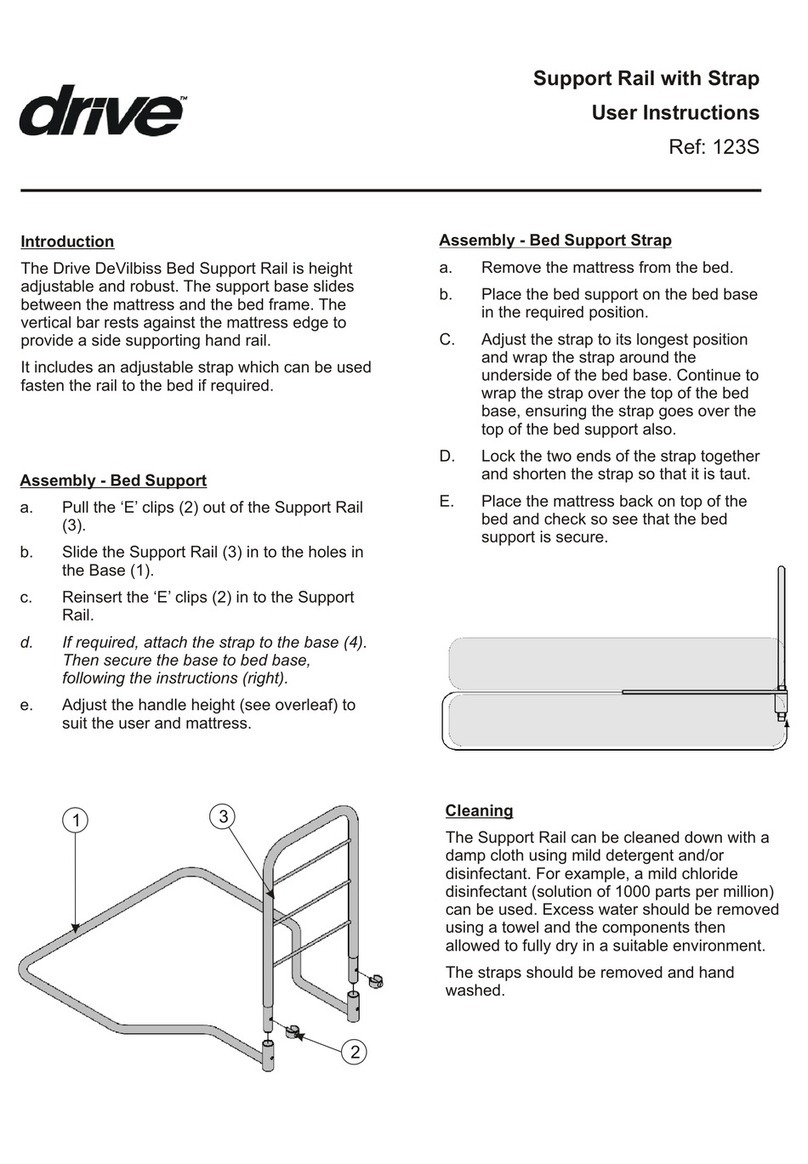
6
LT-2334
GENERAL INFORMATION
WARNING
• This device contains electrical and/or electronic equipment. Follow local governing ordinances and recycling plans regarding disposal of device
components.
MR Unsafe
• Do not bring the device or accessories into a Magnetic Resonance (MR) environment as it may cause unacceptable risk to the patient or damage
to the iGo2 or MR medical devices. The device and accessories have not been evaluated for safety in an MR environment.
• Do not use the device or accessories in an environment with electromagnetic equipment such as CT scanners, Diathermy, RFID and
electromagnetic security systems (metal detectors) as it may cause unacceptable risk to the patient or damage to the iGo2. Some
electromagnetic sources may not be apparent, if you notice any unexplained changes in the performance of this device, if it is making unusual or
harsh sounds, disconnect the power cord and discontinue use. Contact your home care provider.
• This device is suitable for use in home and healthcare environments except for near active HF SURGICAL EQUIPMENT and the RF shielded
room of an ME SYSTEM for magnetic resonance imaging, where the intensity of Electromagnetic DISTURBANCES is high.
• Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is
necessary, this equipment and the other equipment should be observed to verify that they are operating normally.
• Use of accessories and cables other than those specied or provided by the manufacturer of this equipment could result in increased
electromagnetic emissions or decreased electromagnetic immunity of this equipment and result in improper operation.
• Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30
cm (12 inches) to any part of the iGo2, including cables specied by the manufacturer. Otherwise, degradation of the performance of this
equipment could result.
WARNING
THE FOLLOWING BATTERY SAFETY WARNINGS MUST BE OBSERVED AT ALL TIMES:
• Do not drop, hit, crush, or otherwise abuse the battery as this may result in the exposure of the cell contents, which are corrosive.
• Do not subject battery to mechanical shock.
• In the event of a battery leaking, do not allow the liquid to come in contact with the skin or eyes. If contact has been made, wash the affected
area with copious amounts of water and seek medical advice.
• Do not expose the battery to re or extreme heat. Do not incinerate. Exposure of the battery to extreme heat may result in an explosion. Avoid
storage in direct sunlight.
• Do not expose the battery to water, rain or moisture of any type.
• Do not expose to water, re or excessive heat.
• Do not crush, disassemble, puncture or short circuit the connector terminals.
• Do not open, disassemble, or attempt to repair the battery; there are no user-serviceable parts inside.
• Do not short-circuit battery.
• Do not store batteries haphazardly in a box or drawer where they may short circuit each other or be short-circuited by other metal objects.
• Keep batteries out of the reach of children.
• Keep batteries clean and dry.
• Use only the battery in the application for which it was intended.
• Periodically inspect connection cords, connector tips and the power supply for damage or signs of wear. Discontinue use if damaged.
• Charge the battery before initial use.
• Recommended maximum time between charges = 1 year
• Recommendation: Store the battery below 25°C (77°F), low humidity, no dust and no corrosive gas atmosphere. Store fully charged if possible.
• This device contains electrical and/or electronic equipment. Follow local governing ordinances and recycling plans regarding disposal of device
components.
• The battery must be recycled or disposed of properly.
Operating Principle
The Oxygen Concentrator uses molecular sieve to remove the nitrogen from ambient air and allow oxygen to remain in the air delivered to the patient. The
device is a pulse only oxygen concentrator which only delivers oxygen when a patient breath is detected.
Technical Description
Pulse Only Pressure Vacuum Swing Adsorption (PVSA), oxygen concentrator based on molecular sieve technology. Beds are evacuated and drawn down by a
compressor to a vacuum level, which is dependent on setting. Ambient air is then pumped into the concentrator through a series of lters that remove dust and
other particulate. A valve directs air into one of two sieve beds. Nitrogen is adsorbed in the bed as the pressure increases while oxygen ows through, thereby
producing a highly enriched oxygen product. Air is processed in the second bed out of phase in the cycle with the rst bed. This allows processed oxygen to be
used in the cycle and to be delivered to the patient. Oxygen is provided to the patient only with pulse-dose basis.
Healthcare Provider Instructions
• The healthcare provider is responsible to determine oxygen delivery setting for each patient individually with the conguration of the equipment to be used,
including accessories.
• The healthcare provider is responsible to periodically reassess the delivery settings of the oxygen concentrator for the effectiveness of therapy.
• The healthcare provider is responsible to ensure the compatibility of the portable oxygen concentrator and all of the parts used to connect to the patient
before use.