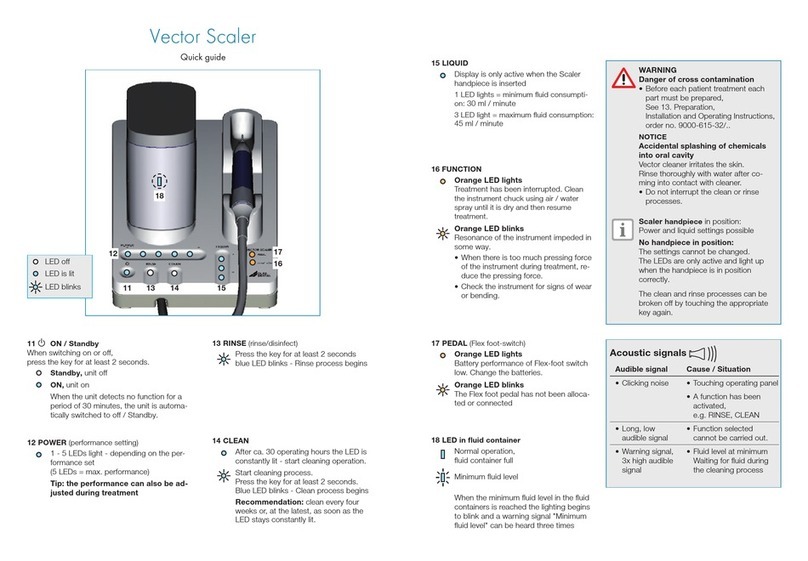
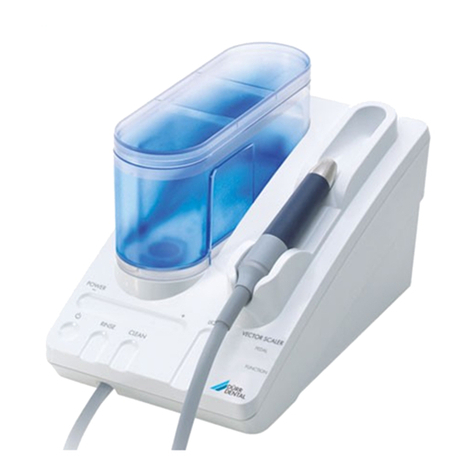
4
Important
Informations
1. Notes
1.1 Conformity Acceptance Procedure
This product has, in accordance with the
European Union directive
93/42/EWG, been checked for conformity and
has been found to fulfill all the requirements of
these regulations.
1.2 General Notes
• These Installation and Operating Instructions
form an integral part of the unit. They must
be kept close to the unit at all times. Precise
observance of these instructions is a pre-
condition for use of the unit for the intended
purpose and for its correct operation.
New personnel must be made aware of the
contents, and they should be passed on to
future operating staff.
• Safety for the operator as well as trouble-
free operation of the unit are only ensured if
use is made of Original Equipment
Manufactured parts. Furthermore, only those
accessories explicitly stated in the Installati-
on and Operating Instructions or
recommended by Dürr Dental may be used.
If other accessories are used, then Dürr
Dental can no longer guarantee safe
operation or smooth functioning of the
appliance. Any claims concerning damage
that arise from the use of other accessories
are inadmissible.
• Dürr Dental are only responsible for the
equipment with regard to safety, reliability
and proper functioning where assembly,
resettings, changes or modifications,
extensions and repairs have been carried
out by Dürr Dental or an agency authorized
by Dürr Dental and if the equipment is used
in conformity with the Installation and
Operating Instructions.
• These Installation and Operating Instructions
conform to the relevant version of the
equipment and the underlying safety
standards valid at the time of going to press.
All switches, processes, trade marks,
software programs and appliances named in
this document are registered names.
• The translation of these Installation and
Operating Instructions has been carried out
in good faith. Dürr Dental denies any liability
for mistakes which might have been made in
the translation. The enclosed German
version of these Installation and Operating
Instructions are considered the original.
• Any reprinting of the technical
documentation, in whole or in part, is subject
to prior approval of Dürr Dental being given
in writing.
• Retain the packaging for possible return of
the product to the manufacturers. Ensure
that the packaging is kept out of the reach of
children. Only the original packaging
provides adequate protection during
transport of the unit.
Should return of the product to the
manufacturers be necessary during the
guarantee period, Dürr Dental accepts no
responsibility for damage occurring during
transport where the original packaging was
not used!
1.3 Appliance Disposal
The CCU-control unit and the other
components contain no batteries of any
type.
The CCU-control unit and the components can
be disposed of as electronic waste. The
heavy metal particles present in the
electronic are classified as dangerous
substances and must be disposed of
according to local and national regulations.
1.4 Notes concerning Medical
Appliances MPG
• This product is a technical medical
appliance and, as such, may only be
operated by trained personnel, or persons
who, as a result of specialist knowledge, are
familiar with this type of appliance.
• Other systems should not be plugged into
the same multi-socket unit.
• Do not plug into any multi-socket unit or any
socket that may be altered locally.