ECHODIA AUDIOSMART User manual

Instructions for use
&
Technical description
Please read these instructions carefully before using your new device!
This manual is an integral part of the device and must be kept until it is
destroyed.
This equipment has been designed and manufactured for use in otologic
diagnosis. Use is restricted to professionals who have undergone appropriate
training.
If you have a problem or do not understand this manual, please contact
your distributor (see stamp on the last page) or contact Électronique du
Mazet at:
Tel: (33) 4 71 65 02 16 - Fax: (33) 4 71 65 06 55
A brand of

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
2
Table des matières
1Information and safety....................................................................................................................................................4
1.1 About this manual....................................................................................................................................................4
1.2 Presentation of the device........................................................................................................................................4
1.2.1
Intended Use ..............................................................................................................................................4
1.2.2
Target population.......................................................................................................................................5
1.2.3
Expected performance ...............................................................................................................................5
1.2.4
Contraindications.......................................................................................................................................5
1.2.5
Side effects.................................................................................................................................................5
1.2.6
Units of measurement ................................................................................................................................5
1.2.7
Accessories ................................................................................................................................................5
1.3 Warnings..................................................................................................................................................................6
1.4 Potential risks ..........................................................................................................................................................7
1.4.1
Shutdown of the device during its operation..............................................................................................7
1.4.2
Special use case..........................................................................................................................................7
1.5 Commissioning........................................................................................................................................................7
1.5.1
Charging the device ...................................................................................................................................7
1.6 Applicable symbols .................................................................................................................................................8
1.7 Identification label...................................................................................................................................................9
1.8 Patient data confidentiality ....................................................................................................................................10
1.9 Cybersecurity.........................................................................................................................................................10
1.9.1
Good practices for computer security ......................................................................................................10
1.9.2
Technical Information..............................................................................................................................10
2General information about using AUDIOSMART....................................................................................................11
2.1 Initial Start-up of the device ..................................................................................................................................11
2.1.1
Switching on / starting .............................................................................................................................11
2.1.2
Touch screen calibration..........................................................................................................................11
2.1.3
Password..................................................................................................................................................11
2.1.4
Home screen ............................................................................................................................................12
2.1.5
Switching off the device ..........................................................................................................................12
2.2 General device configurations..............................................................................................................................13
2.2.1
Selection of the connected Jack headphone.............................................................................................14
3Introduction and test setup...........................................................................................................................................15
3.1 Equipment..............................................................................................................................................................15
3.1.1
Setup ........................................................................................................................................................16
4Handheld mode measurement......................................................................................................................................17
4.1 Patient management..............................................................................................................................................17
4.1.1
Create a new patient.................................................................................................................................17
4.1.2
Patient follow-up......................................................................................................................................18
4.2 Audiometry............................................................................................................................................................19
4.2.1
Pure-tone Audiometry..............................................................................................................................19
4.2.2
High frequency audiometry .....................................................................................................................22
4.2.3
Speech audiometry...................................................................................................................................23
4.2.4
Measurement consultation .......................................................................................................................25
5The use of the software ECHOSOFT ..........................................................................................................................26
5.1 Minimum configuration needed ............................................................................................................................26
5.2 Installation.............................................................................................................................................................26
5.2.1
Installing the software..............................................................................................................................26
5.2.2
Installation des pilotes USB.....................................................................................................................27
5.3 Patient management...............................................................................................................................................27
5.3.1
Create new patient....................................................................................................................................28
5.3.2
Import patient from device.......................................................................................................................28
5.3.3
Delete a patient ........................................................................................................................................30
5.4 Settings..................................................................................................................................................................31
5.4.1
Database...................................................................................................................................................31

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
3
TABLE DES MATIÈRES
TABLE DES MATIÈRES
5.4.1
Medical software......................................................................................................................................32
5.4.2
Puretone audiometry settings...................................................................................................................32
5.4.3
Impression................................................................................................................................................33
5.5 Update ...................................................................................................................................................................33
5.5.1
AUDIOSMART update........................................................................................................................34
5.6 Audiometry with ECHOSOFT ............................................................................................................................34
5.6.1
Pure tone audiometry...............................................................................................................................35
5.6.2
Speech audiometry...................................................................................................................................36
5.7 Exploitation sur ECHOSOFT................................................................................................................................37
5.7.1
Opening a measurement...........................................................................................................................37
5.7.2
Description of the measurement window.................................................................................................38
5.8 Masking calculation help.......................................................................................................................................39
5.8.1
Color code................................................................................................................................................40
5.8.2
« Automatic mode » audiometry with « Auto mode » masking ..............................................................40
5.8.3
Calculation method..................................................................................................................................40
5.9 Merge measurement ..............................................................................................................................................41
5.10 Using keyboard shortcuts ......................................................................................................................................42
5.11 Use of the microphone...........................................................................................................................................42
6Maintenance and servicing...........................................................................................................................................43
6.1 Periodic checks......................................................................................................................................................43
6.2 Cleaning.................................................................................................................................................................43
6.2.1
Device case ..............................................................................................................................................43
6.2.2
Accessories ..............................................................................................................................................44
6.3 Malfuction .............................................................................................................................................................44
6.3.1
Possible malfunction................................................................................................................................44
6.3.2
After-sales service and warranty..............................................................................................................45
6.4 Transport and storage ............................................................................................................................................46
6.5 Disposal.................................................................................................................................................................46
7Technical specifications ................................................................................................................................................47
7.1 General technical characteristic of the device .......................................................................................................47
7.1.1
Test parameters :......................................................................................................................................48
7.2 Standards/Certifications.........................................................................................................................................49
7.2.1
EMC compliance table.............................................................................................................................49
7.2.2
CE declaration..........................................................................................................................................51
7.3 Manufacturer .........................................................................................................................................................51

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
4
Chapter 1
Information and safety
1.1
About this manual
This user and maintenance manual is published to help you to get started with your AUDIOSMART device from the
initial receipt, through commissioning, use and maintenance.
If you have any difficulty in understanding this manual, contact your dealer/distributor or the manufacturer, Élec-
tronique du Mazet.
This document must be kept in a safe place, protected from atmospheric agents, where it cannot be damaged.
This document ensures that the devices and their documentation are technically up-to-date at the time of marketing.
However, we allow ourselves to make changes on the device and its documentation without any obligation to update
these documents.
In the case of transfer of the device to a third party, it is mandatory to notify Électronique du Mazet about the new
owner information’s. The device must be provided to the new owner with all documents, accessories and packaging.
Only staff aware of the content of this document are allowed to use the device. If the instructions contained in this
document are not followed, Électronique du Mazet and its distributors disclaim all responsibility about consequences of
accidents or damage on staff or third parties (including patients).
1.2
Presentation of the device
AUDIOSMART is designed for screening, documentation, monitoring and diagnosis of hearing functions. It is intended
for ENT doctors, audiologists, pediatricians and other health professionals practicing in private or in a hospital environ-
ment. The audiometry is a behavioral examination allowing to quickly assess the hearing acuteness. Via an acoustic
stimulator, sounds, words or sentences with various sound intensities are presented to the subject. The subject reports his
or her perception to the operator who can, according to the test used, determine an absolute threshold of perception or an
intelligibility threshold.
1.2.1
Intended Use
The audiometric diagnostic test is a behavioural test for the rapid assessment of hearing ability. The subject is exposed
to sounds, words or phrases at different intensities using an acoustic stimulator. He/she transmits his/her perception to the
operator who, depending on the test used, can detect a reduction in hearing acuity, determine an absolute threshold of
perception or an intelligibility threshold. Two modes of transduction can be used, through the normal auditory pathways
using an acoustic transducer (Air conduction), or using a vibrator placed on a bony part such as the mastoid or the forehead
(Bone conduction).
AUDIOSMART is designed to perform the following otologic diagnostics :
Audiometry:
-Air conduction (AC)
-Bone conduction (BC)
-Speech
User guide AUDIOSMART
ECH001XN141-A4 –07/2022
5
Chapter 1. Information and safety 1.2 Presentation of the device
1.2.2
Target population
Ages: The device can be used on any type of patient with the ability to respond to the presence or absence of an
acoustic stimulus (>5 years)
Patient type: men / women / children / newborn
Consultation context: ENT diagnosis & occupational medicine
1.2.3
Expected performance
The devices are designed to perform otologic tests according to ISO 60645 standards:
Otologic tests
Standards
Audiometry:
-Air conduction (AC)
-Bone conduction (BC)
IEC 60645-1 :2017 - Type 3
Compatible EHF
- Speech
IEC 60645-1 :2017 - Classe B
1.2.4
Contraindications
We recommend not to diagnose (or to take precautions when diagnosing) patients with injured skin, open wounds or
acoustic hypersensitivity
The contraindications are not exhaustive and we advise the user to seek advice in case of doubt.
1.2.5
Side effects
No side effects identified to date
1.2.6
Units of measurement
For all these devices, the units of measurement are expressed in the units of the international system:
Base unit
Unit
Name
Symbol
Frequency
Hertz
Hz
Voltage
Tension
V
Intensity (Decibel)
Sound pressure level
Hearing level
dB SPL
dB HL
1.2.7
Accessories
This device is delivered with the following accessories as standard:
- Mini-USB cable 2m
The device is in contact with the patient through applied parts, some of them are supplied by Electronique du Mazet.
These accessories can be single use or reusable.
The use of accessories not recommended by the manufacturer does not engage his responsibility
List of compatible accessories:
Name
ref
Manufacturer
DD45 headset
301765
Radioear
DD65 headset
301475
Radioear
DD450 headset
302427
Radioear
Insert Earphones
040070
Electronique du Mazet
Bone vibrator B71
040060
Electronique du Mazet
Patient response switch
040084
Electronique du Mazet
Mini-USB cable 2m
300618
Lindy
USB power adapter (EU)
301526
CUI

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
6
Chapter 1. Information and safety 1.3 Warnings
USB power adapter (USA)
040048
CUI
USB power adapter (UK)
040047
CUI
Foam earplug ER3-14A 13mm (50 pcs)
40116
3M
Foam earplug ER3-14B 10mm (50 pcs)
40117
3M
1.3
Warnings
In this manual the warnings and information given have the following meaning:
The caution label indicates conditions or process that may expose the patient and/or user to a risk.
The warning label indicates conditions or process that could cause the device malfunction.
The information label refers to notices or information that are not related to any risk of accidents or
malfunction of the device.
CAUTION: The device must be handled by a qualified operator (hospital staff, doctor, etc.). The
patient must not come into contact with the device other than through the accessories.
CAUTION: The device must be connected to a computer with a certified medical power supply
(double insulation according to ISO 60601-1)
CAUTION: No modifications to the device are permitted. It is strictly forbidden to open the device
housing.
CAUTION: This equipment complies with applicable electromagnetic compatibility standards. If
you experience interference or other problems with another device, contact Électronique du Mazet
or the distributor for advice on how to avoid or minimize the problem.
CAUTION: Operation in close proximity (e.g., 1 m) to shortwave or microwave therapy EM equip-
ment may cause instabilities in the output power of the STIMULATOR
CAUTION: The device must be used with accessories given compatible by the manufacturer (see
Erreur ! Source du renvoi introuvable.).
CAUTION: The device must not be accessible to the patient.
It should not be placed in contact with the patient.
CAUTION: the computer must never be located in a space accessible to the patient
CAUTION: Be sure to follow the maintenance instructions listed in the 6.Maintenance and servi-
cing
User guide AUDIOSMART
ECH001XN141-A4 –07/2022
7
Chapter 1. Information and safety 1.4 Potential risks
CAUTION: The battery can only be replaced by Électronique du Mazet technicians or their distrib-
utors.
The device collects data. The practitioner is in charge to comply with the EU General Data Protection
Regulation 2016/679 (or the local laws of the personal data protection). When returning to the After
Sales Service, the practitioner must delete the data so that it is not disclosed.
1.4
Potential risks
Applied parts that are too old or of poor-quality can impair the quality of contact with the patient and cause discomfort.
Make sure to regularly change the parts.
Microbes or viruses can be transmitted from one patient to another via the applied parts. Make sure that the hygiene
conditions recommended by the manufacturer of the applied part are observed.
If water enters the device, it may not function properly. In this case, unplug the device and disconnect the cables. In
any case, avoid the presence of water in the vicinity of the device.
1.4.1
Shutdown of the device during its operation
In case the device is shutdown during its operation,
- In stand-alone mode: the measurement in progress will stop; the continuous saving of the measured data avoids
losing the measurements made up to that point.
- When connected to the computer: the computer continuously saves the data, the measurement can be saved before
closing the software.
1.4.2
Special use case
No specific cases identified. See section Erreur ! Source du renvoi introuvable. for contraindications.
1.5
Commissioning
Check that the device is not damaged; if you have any doubts about the integrity of the device and its proper func-
tioning, contact Électronique du Mazet or your distributor.
If the device was stored in a cold place and there was a risk of condensation, let the device rest for at least 2 hours at
room temperature before switching it on.
Before using the device for the first time, cleaning it and its accessories is recommended, see 6.Maintenance and
servicing
1.5.1
Charging the device
The device is delivered with a USB cable. You can choose between two ways of charging your device, via a computer
or via the USB power (see Erreur ! Source du renvoi introuvable.). Once plugged in, the charge starts automatically
and an electrical plug logo is displayed in the title bar. This logo appears in grey when the AUDIOSMART is charging and
in green when the battery is fully charged.
The device battery is charged before shipment; however, it is recommended to charge it before the first use (we advise
you to charge it for 12 hours before the first use).
When using the solution of connecting the device to a computer via the USB cable, charging will be slower than via
a USB power adapter (see Erreur ! Source du renvoi introuvable.).
It is preferable to charge/discharge the battery as fully as possible to ensure a long service life. Charge the
device to its maximum capacity and only charge it when it has reached a critical battery level.
To disconnect the device from the power supply, the USB power adapter must be disconnected.
User guide AUDIOSMART
ECH001XN141-A4 –07/2022
8
Chapter 1. Information and safety 1.6 Applicable symbols
1.6
Applicable symbols
Front side
Name of the device
Upper side
Caution: Switching the device on/off
USB
Mini-USB port for recharging the device, or connection to a PC (data exchange)
Lower side
AUX
-Patient answer button connection for audiometry
-EchoDif connection for electrophysiology
Audio
-Acoustic stimulator connection for audiometry and electrophysiology
-OAE probe connection for otoacoustic emissions
Headset connection
Back side
Warning: this logo draws your attention to a specific point
Operating instructions: this logo informs you that the operating instructions must be
read for safe use of the device
BF type applied part: applied parts not supplied by Electronique du Mazet are in elec-
trical contact with the patient, floating and not connected to earth.
Recycling: This device should be disposed of at an appropriate collection and recycling
facility. Consult the manufacturer.

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
9
Chapter 1. Information and safety 1.7 Identification label
Direct current
Serial number
Manufacturer
Year of manufacture
Country of production
Product reference
CE marking
1.7
Identification label
Information and specifications are given on the back of each device on an identification label:
Device :
Device identification label
AUDIOSMART
ECH001KP140-A0

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
10
Chapter 1. Information and safety 1.8 Patient data confidentiality
1.8
Patient data confidentiality
The device collects data. It is the practitioner's responsibility to apply and comply with the European Parliament's
General Data Protection Regulation 2016/679. When returning the device to the after-sales service, the user must delete
the patient data from it so that it is not disclosed. The practitioner has the possibility to make a backup copy of the data
by saving them in the ECHOSOFT software (see section Erreur ! Source du renvoi introuvable.)before deleting the
patients from the device (see section Erreur ! Source du renvoi introuvable.).
The AUDIOSMART device is intended to be used by authorized healthcare professionals only. To ensure the confiden-
tiality of patient data and to prevent disclosure to unauthorized third parties, a password can be set at the first start of the
device. Please refer to section Erreur ! Source du renvoi introuvable. for more information.
1.9
Cybersecurity
As the device and its ECHOSOFT software are computer-based systems that are integrated into larger information
systems, certain rules and good practices must be put in place to ensure the safety of patients and users.
Électronique du Mazet does not provide or control the operating environment of its products, so it is the responsibility of
the practitioner to ensure that the following recommendations are followed.
1.9.1
Good practices for computer security
-Keep your software up to date, including the operating system (Windows or MacOs)
-Use operating system accounts to manage access.
-Use strong passwords to access accounts
-Lock down the computer when not in use
-Back up the ECHOSOFT database regularly (see 5.4.1)
-Verify the authenticity of any third-party software you install
-Use anti-virus software and a firewall
-Since the device and ECHOSOFT do not need to access the Internet, isolate the computer from the network as much as
possible.
-Check echodia.com periodically to see if updates are available.
1.9.2
Technical Information
-The ECHOSOFT software is a Java program
-It embeds its own java execution environment (JRE+JVM) in order not to interfere with other software. (installed in the
same folder, by default : C:\Program Files\Echodia\Echosoft\jre)
-The configurations of the software as well as the database are saved in the .echosoft folder of the user folder (ex: C:\Us-
ers\romain\.echosoft).
-The software uses the port 32145 of the local loop (localhost / 127.0.0.1) in order to check that there are not several in-
stances of the software launched at the same time.
-The software uses a proprietary USB driver to communicate with the device
ECHODIA advises you to regularly renew the password of your device. It is also advisable to activate the
lock mechanism of the computers on which you have installed the ECHOSOFT software after a short
period of inactivity.

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
11
Chapter 2
General information about using AUDIOSMART
2.1
Initial Start-up of the device
2.1.1
Switching on / starting
The device can be turned on without any other part connected.
Turn on the power using the switch on top of the device (if it does not start, make sure the device's battery is charged)
2.1.2
Touch screen calibration
For the first start-up, it is necessary to calibrate the touch
screen. The following window appears:
The screen has to be calibrated at 5 different points.
Simply hold the stylus down on the cross at the center
of each of the circles that appear successively.
2.1.3
Password
After the screen calibration, the password definition windows appear. If you choose to set up a password, it will be
asked to you each time you start the device. To do this, click on “Lock the device with a password” and set your password
by clicking on “Change Password”. The password must contain between 1 and 15 characters, and will be asked twice in
order to ensure the proper seized of it.
You can access the password configuration window later from the “Measure” then “System” menu. This window
allows you to change the password, but also to activate or deactivate the locking.
If you forget your password, please contact Electronique du Mazet or your reseller to receive an unlocking code.
Calibration is important for comfortable use. It is highly advisable to calibrate the device while holding it on
a table, and using the stylus.
User guide AUDIOSMART
ECH001XN141-A4 –07/2022
12
Chapter 2. General information about using AUDIOSMART 2.1 Initial Start-up of the device
2.1.4
Home screen
Once this step completed, the homepage appears:
Several items of information appear on this page. First, it contains the 3 possible selection parameters when the device
is starting:
•
USB: enables to activate the USB port of the device in order to recover, store and analyze the measurements made
with it on a computer. Enabling the USB port of the device is also necessary for the realization of measures from a
computer via the ECHOSOFT software.
•
Measure: this is the main mode used for making and consulting measurements.
•
Config: configuration of the various device options.
The homepage is used for choosing the system language by clicking on the flags at the bottom left of the screen.
At the bottom right, the serial number of your device is displayed.
A title bar is present on all windows of the device. From left to right, it contains:
•
The current window title.
•
The charge indicator (grey: charging. Green: charged).
•
The date and time.
•
Tha battery level.
•
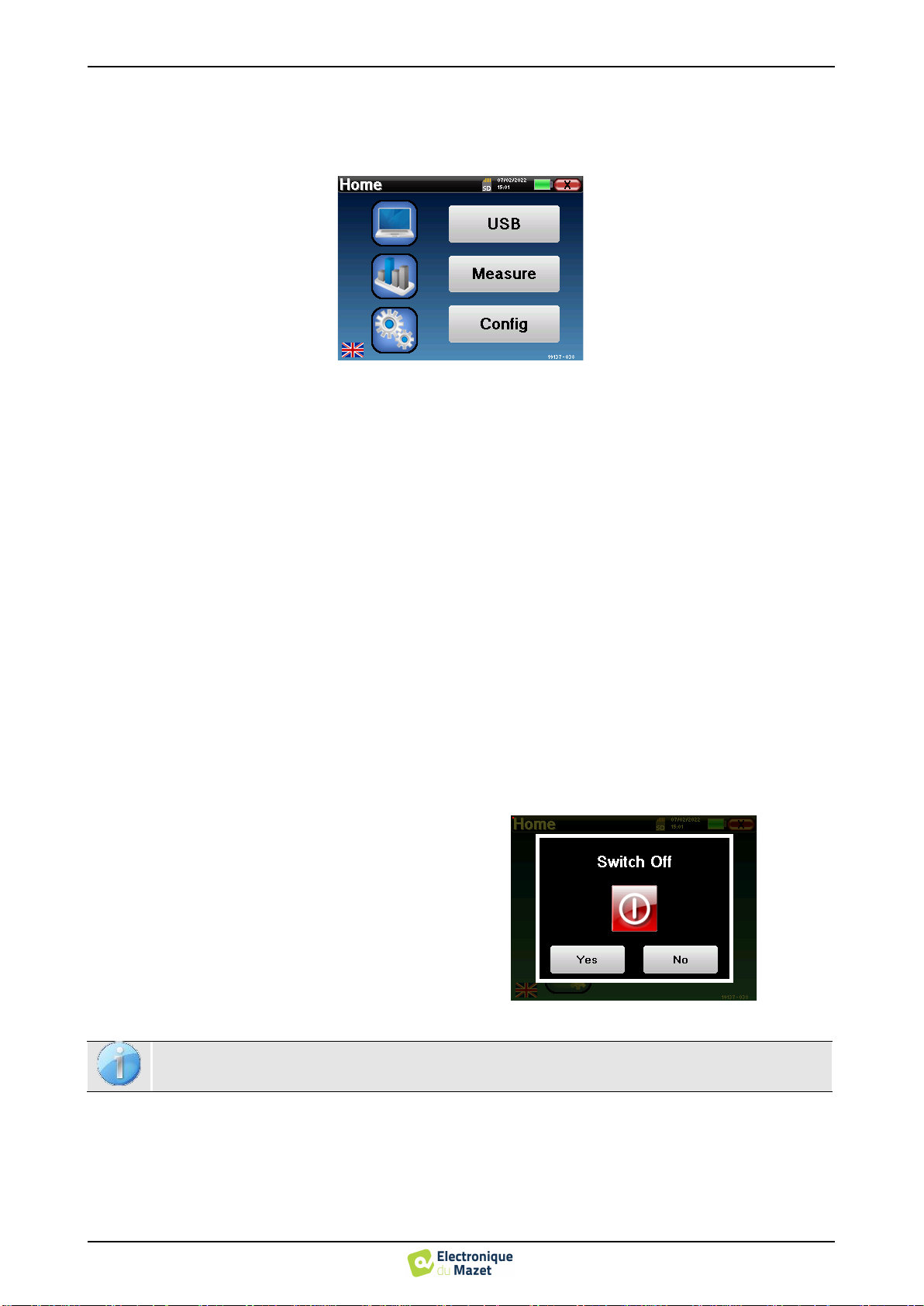
A button for returning to the previous window (on the homepage screen, it is used for switching off the device).
2.1.5
Switching off the device
To turn off the device, you can click on the back button
at the top right of the home screen. A shutdown confir-
mation message will appear:
It is also possible to press the power button on the top
of the device to bring up this screen from any browser
window.
Energy saving mode: when you are not measuring, the
device automatically turns off after 5 minutes of inac-
tivity.
.
It is possible to force the device to turn off by holding down the power button at the top of the device dur-
ing 4 seconds.

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
13
Chapter 2. General information about using AUDIOSMART 2.2 General device configurations
2.2
General device configurations
Some of the device general operating parameters can be
configured. Accordingly, it is possible to set the time,
date, brightness and orientation of the screen. To do this,
just enter the configuration menu from the main screen.
The date and time can be configured from the "Date
and Time" window
The "LCD" menu allows you to adjust the brightness
of the screen with an adjustable gauge. The "Rotation"
button allows the display to be rotated by 180°. This can
be useful depending on the location and position in
which the device is used. It is also possible to recalibrate
the touch screen.
The "System" button informs you about the hardware
and software version of the device, and the amount of
free memory space on the AUDIOSMART device.
The «Data factory reset » button resets the measure-
ment parameters to the default values.
If you choose to set up a password lock, you will be
asked for it every time you start the device (see Erreur !
Source du renvoi introuvable.).
The "Settings" button enables the access to the menu
for activating the optimised start-up modes for operators
who use (mainly) the device connected to the computer
(ECHOSOFT). The "Settings" button enables the access
to the menu for activating the optimised start-up modes
for operators who use (mainly) the device connected to
the computer.
The change to and from daylight saving time is not automatic.
It is possible that some drifting appears after using the tactile screen for some time (several months) (e.g.:
clicking on the buttons becomes less accurate), in which case screen re-calibration is necessary.
User guide AUDIOSMART
ECH001XN141-A4 –07/2022
14
Chapter 2. General information about using AUDIOSMART 2.2 General device configurations
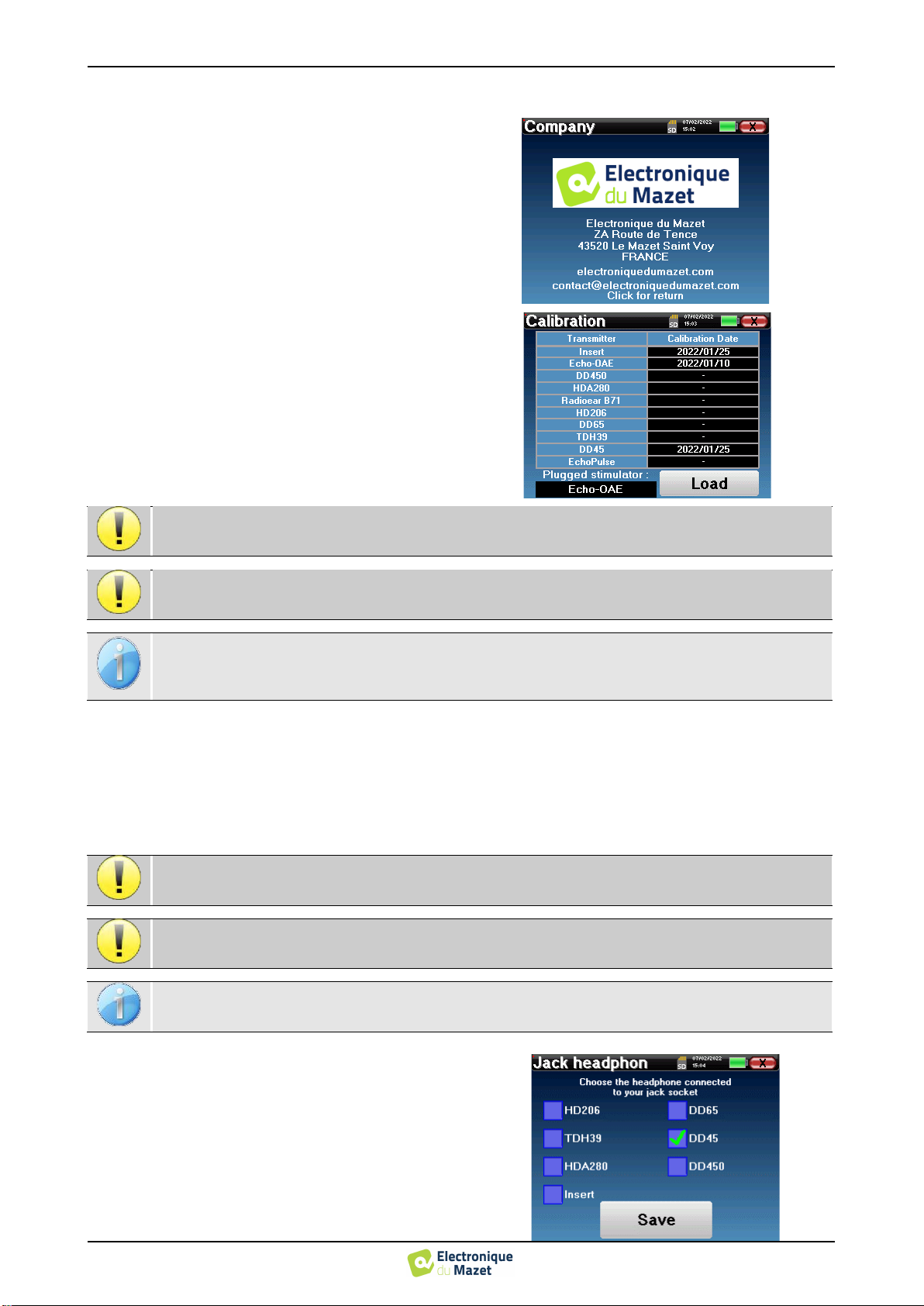
The «About» menu contains the contact details of the
company Electronique du Mazet.
The “Calibration” menu allows the consultation of the
acoustic calibration values defined for your device.
2.2.1
Selection of the connected Jack headphone
In most cases, the unit comes with only one headset, which is properly configured at the factory. However, you have
the option of changing the type of headphones that will be recognized when connected to the jack socket. If you have
several headphones with a jack plug that have been calibrated for your device, you will need to go through this menu to
switch from one to the other.
In the main menu of the unit, click on "Config". The
settings window will open. Click on "Headset" to access
the selection of the headset that will be recognized when
connected to the jack. Select the headset model you will
use and click "Save".
Don't modify this value, only Electronique du Mazet or your reseller are approved to realize this cali-
bration.
The AUDIOSMART device must be calibrated once every year to guarantee the quality of the measure-
ments. Please contact your retailer to plan this calibration.
Some of these options require a password to be changed. This is the serial number of your device, indi-
cated on the back of it on the S/N line. This number is also displayed at the bottom right of the home
page.
Never connect headphones that have not been calibrated for your device!
It is extremely important to choose the right model of headphone to ensure that the calibration is correctly
loaded when using it.
Stimulators connected to the "Audio" input are automatically recognized by the device.

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
15
Chapter 3
Introduction and test setup
Audiometry is the basic review of hearing. This test allows a quick check of hearing, from the transmission chain to
the brain. The measure is obtained through the emission of a calibrated frequency sound wave, of which we will lower
the level until the patient can't hear it anymore. The sounds are emitted by a supra-aural headphone, in one ear then in the
other.
Pure-tone Audiometry allows hearing thresholds screening for each ear in a frequency range from 125Hz to 8 kHz with
regular headset and up to 16KHz with high frequency headset. Bone conduction evaluates performance of the inner ear
and the auditory nerve, air conduction tests all of the acoustic function from the external ear to the auditory nerve.
The interpretation of the resulting audiogram allows to know the degree of hearing loss and the kind of deafness.
Pure tone audiometry also allows to find a discomfort threshold, or also the research for any tinnitus frequency.
Speech audiometry is a further investigation of the pure-tone audiometry. It does not determines a threshold of
perception, but a threshold of intelligibility of language, or discernment of phonemes. The test consist of making the
patient repeat a series of words he hears. By varying the power of diction of the words, a curve relating the percentage of
discrimination in relation to the power is obtained. Well known by hearing aid specialist to fine tune the settings of hearing
aids, it is also used to diagnosis retrocochlear pathologies like neuropathy or acoustic neuromas
3.1
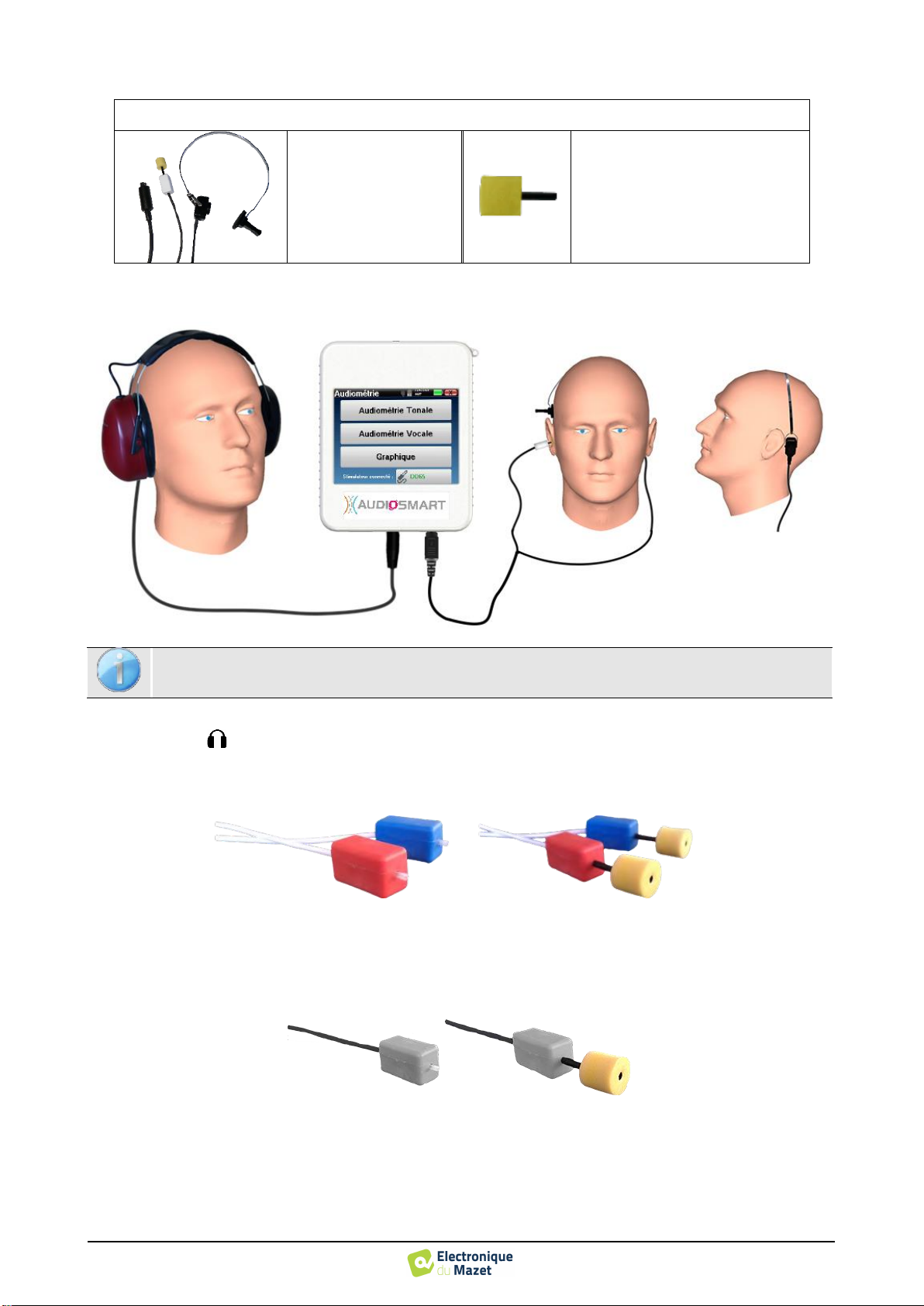
Equipment
To make a Audiometry measurement you need the following equipment:
Common elements for all configurations
AUDIOSMART unit
Air conduction audiometry
1 audiometric headset or the insert earphone
User guide AUDIOSMART
ECH001XN141-A4 –07/2022
16
Bone conduction audiometry
B71 bone vibrator
2 foam ear tips ER3-14A 13mm
or
2 foam ear tips ER3-14B 10mm
3.1.1
Setup
•
To make measurement with headset, connect the cable on the Jack plug of the AUDIOSMART (indicated with the
headset icon ).
•
For measurement with the insert earphone, put the tip on the left and right earphones. Then, connect the ear-
phone Mini-DIN to the «Audio» connector of the AUDIOSMART unit.
•
For bone conduction measurement, put the vibrator on the mastoid (or on the forehead for Webber test)
and put a foam ear tip on the contralateral masking insert earphone. Then, connect the earphone Mini-DIN
to the «Audio» connector of the AUDIOSMART unit.
•
Explain to the patient the procedure for performing an audiometry.
•
Put the headset on the head of the patient.
Using an otoscope, make sure that the ear canal is not obstructed by ear-wax.
This operation must be carried out by a qualified person.

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
17
Chapter 4
Handheld mode measurement
4.1
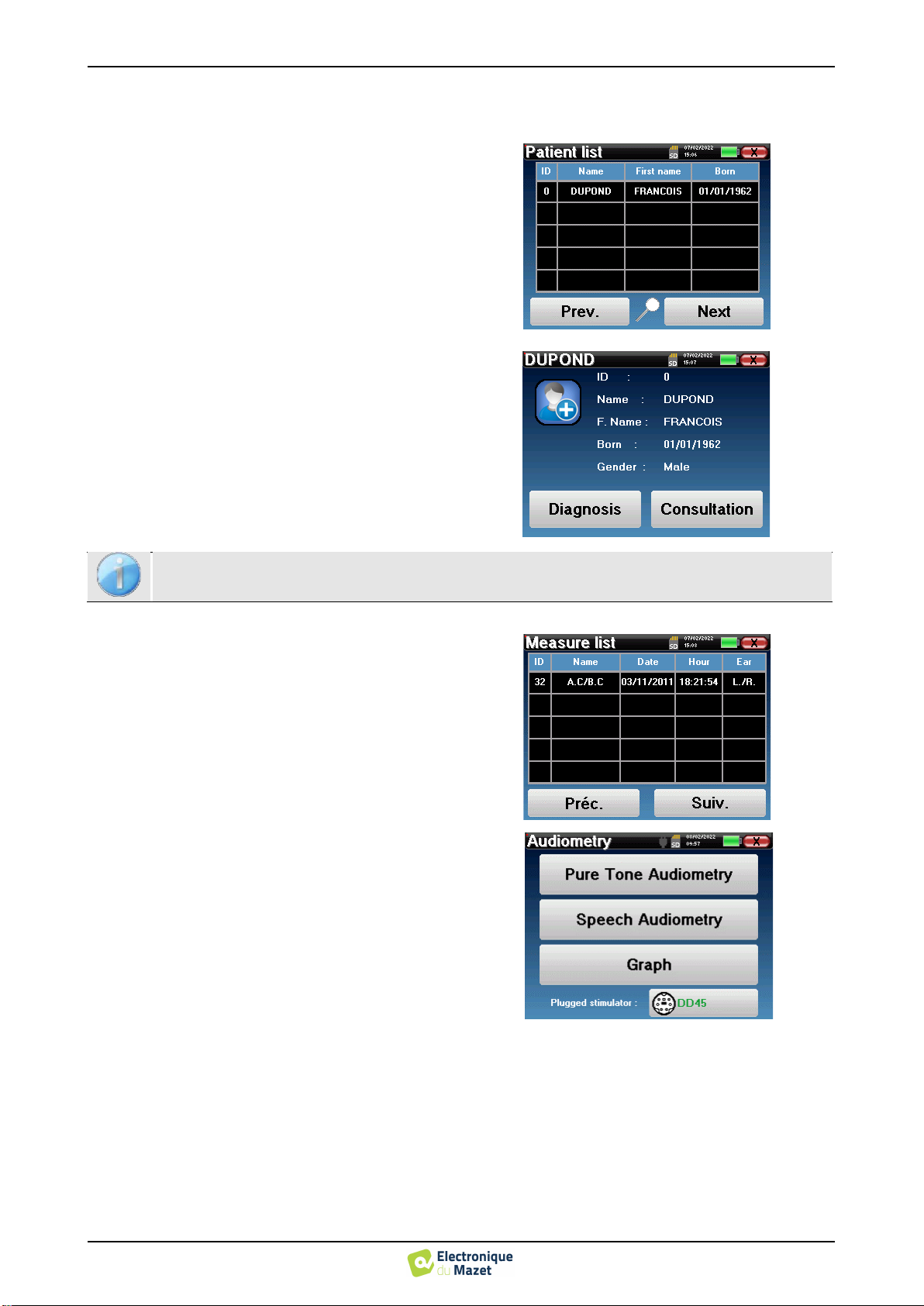
Patient management
AUDIOSMART offers ideal measurement organization
thanks to an advanced patient-based management sys-
tem.
From the home page, select the "Measure" mode, lead-
ing to the choice of seeking an existing patient or creat-
ing a new one.
4.1.1
Create a new patient
If you choose to create a new patient, only 4 items of
information are requested, the name, first name, date
of birth and gender.
To enter this information, just click on the desired field
to have the keyboard appearing on the screen.
It is possible to use a digital keypad by clicking on the
"123" key at the bottom left.
Here, the information about the patient is kept brief. You can enter more details when you export the data to the
ECHOSOFT program. Refer to paragraph 3.2
By entering the patient's date of birth and gender, it is possible to plot the audiometric normals.
To create a new patient, it isessential to specify a name and a first name. It isalso recommended to specify a
birth date, this allows a better patient organization in the ECHOSOFT data base.
The date must be entered in format DD/MM/YYYY (day/month/year). AUDIOSMART device automatically
formats your enter.
.
User guide AUDIOSMART
ECH001XN141-A4 –07/2022
18
Chapter 4. Handheld mode measurement 4.1 Patient management
4.1.2
Patient follow-up
After you have created the patient, his or her record is
recorded on the memory card. You can then find it by
clicking on the "Search" button.
This brings up a table containing the list of patients, in
the opposite order to the recording (the last added pa-
tient appears at the top of the list).
The list of patients appears with names, first names and
dates of birth. The magnifying glass icon at the bottom
of the screen enables to search for a patient thanks to
his/her name or surname.
To select a patient, click on the corresponding line.
This opens a new page summarizing information about
the patient.
Now, it becomes possible to choose to make a new
measurement (diagnosis) or to consult those previously
saved (consultation).
The "Consultation"button opens a table of measure-
ments allowing to consult the diagnoses previously
made for this patient.
To find the desired measurements, the main information
is displayed (type, date, time and ear).
The "Diagnosis" button allows to start a new measurement.
If the patient does not yet have a measurement, only the «Diagnosis»button is visible.

User guide AUDIOSMART
ECH001XN141-A4 –07/2022
19
Chapter 4. Handheld mode measurement 4.2 Audiometry
4.2
Audiometry
Refer to paragraph Erreur ! Source du renvoi introuvable. to obtain the necessary instructions about the needed
equipment and the setup.
When you start a new diagnosis, the configuration window
appears. It allows to start new measurement of pure-tone
Audiometry or speech Audiometry. The “Graph” button al-
lows you to view the graph at any time of the current meas-
urement. The last button shows the active stimulation and can
be used to switch between the two audio output. Thus, it is
possible to keep the audiometric headset and the bone vibra-
tor connected and switch between them by clicking on this
button.
4.2.1
Pure-tone Audiometry
Once you select the Pure Tone Audiometry test you
can choose among four modes of diagnosis.
- Automatic patient mode.
- Automatic doctor mode.
- Manual doctor mode.
- Weber Mode.
4.2.1.1 Patient mode
The patient mode allows automatic transitions of powers and frequencies. The doctor have to pre configure the test,
then the patient is completely autonomous, he simply must click on the device to indicate that he hears the sound.
Measurement settings
Click on “Settings” then “Frequencies selection” in order to preconfigure the frequencies to run during the test. Once
they are chosen, click on “OK” to validate.
All frequencies can be selected, however, the maximum and minimum stimulation frequencies may be
limited when making measurement depending on the characteristics of the stimulator.
The icon of a floppydisk at the bottom right of the screen is used for recording the parameters defined above.
They then become the default parameters for this type of measurement.
Table of contents
Other ECHODIA Diagnostic Equipment manuals