R-720-190 Rev. E English 4
1. Important Information
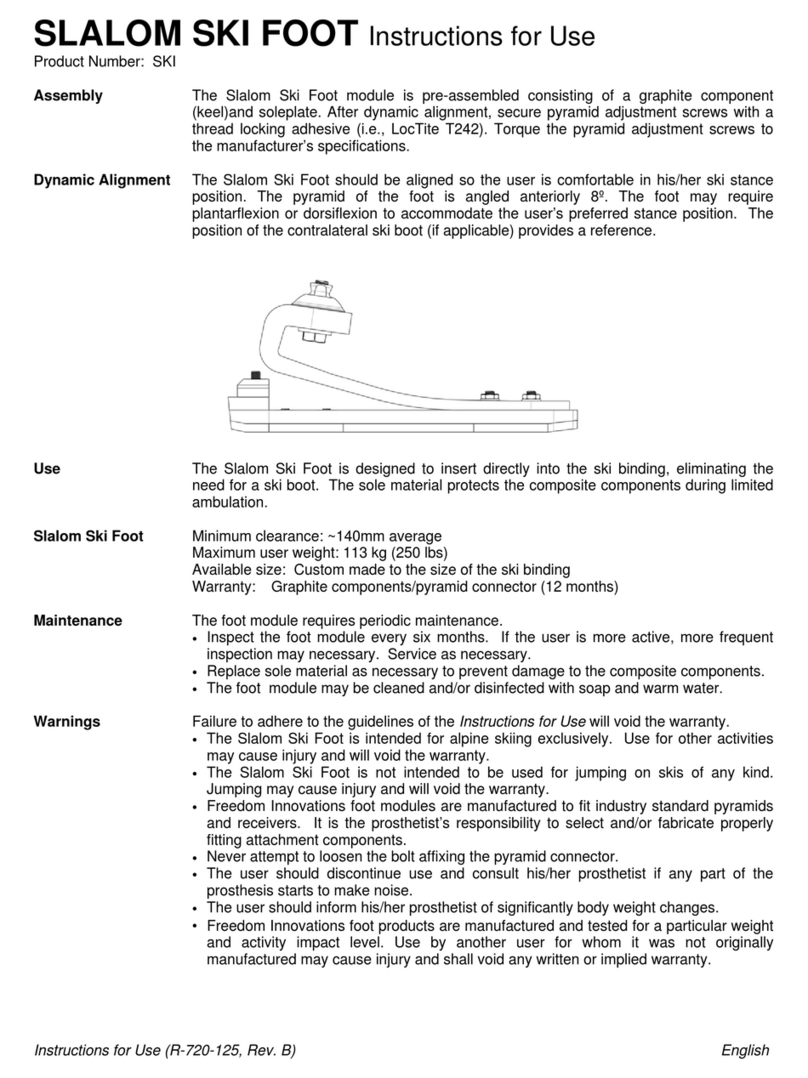
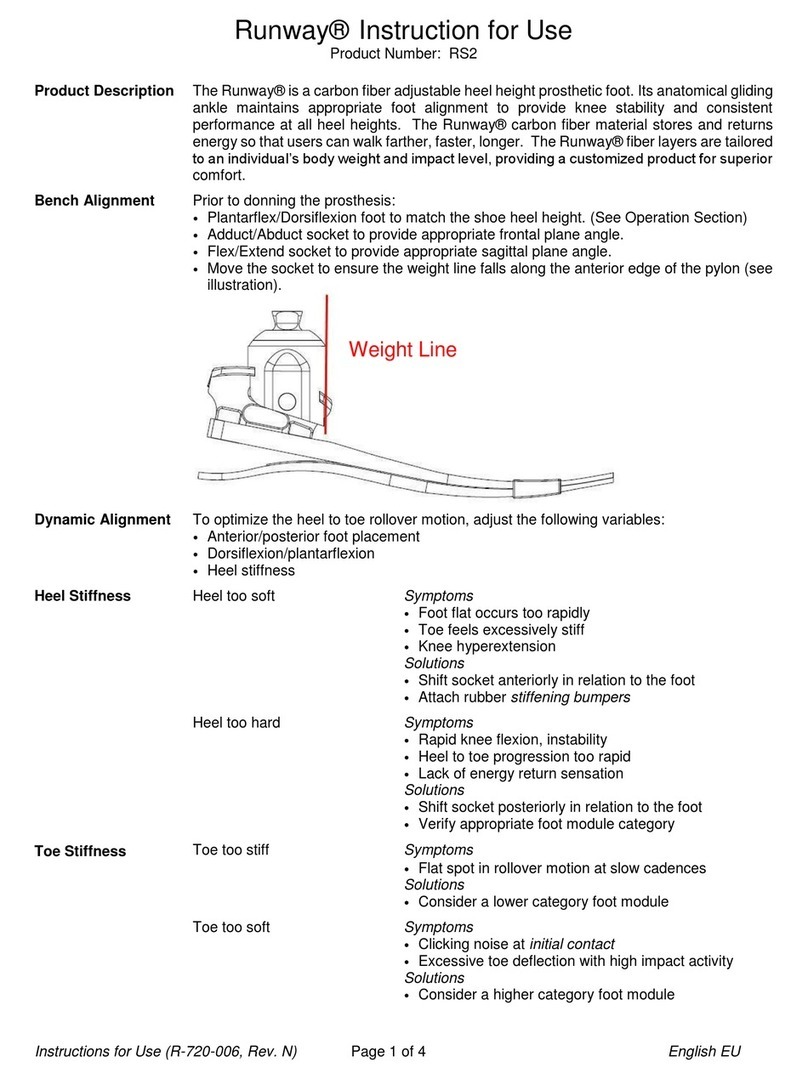
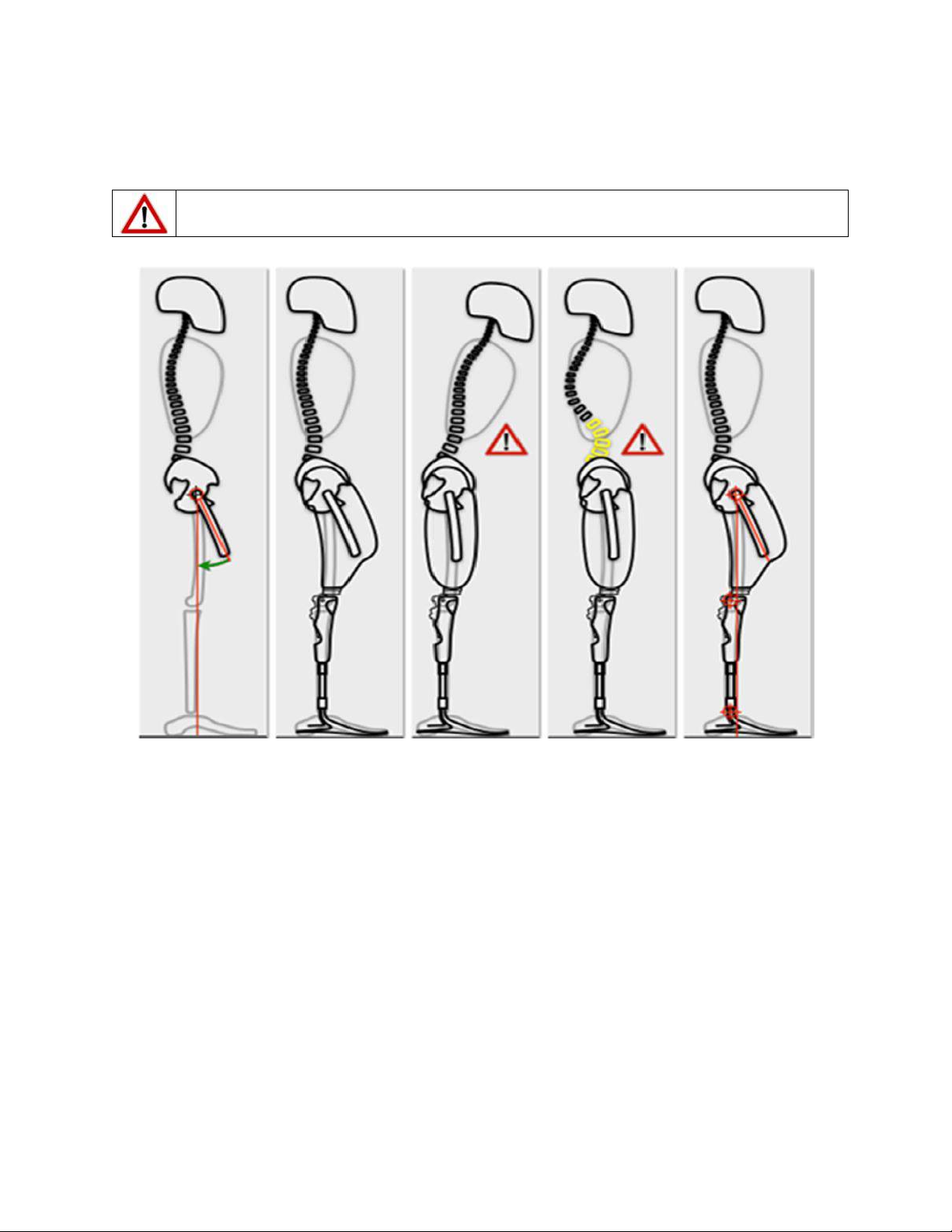
The Plié® 3 MPC Knee is a single axis prosthetic knee joint system providing microprocessor
control of both the swing and stance phases of gait. The microprocessor monitors an
embedded load sensor and an angle sensor to precisely control the transitions between the
stance and swing phases of gait. Three manual settings allow the hydraulic cylinder to provide
adjustable resistance for Stance Flexion, Swing Flexion, and Swing Extension. The hydraulic
cylinder also provides non-adjustable stance extension resistance. The Plié Control software
allows the knee function to be optimized for each individual user’s gait, including the stumble
recovery parameters. The Gait Lab software provides the prosthetist with access to recorded
data files of the microprocessor for analysis and documentation.
1.1 Indications
The Plié® 3 MPC Knee is intended for use as a component in a prosthetic leg for individuals with
lower-limb loss including:
•transfemoral amputees
•knee disarticulation amputees
•hip disarticulation amputees
•individuals with congenital lower-limb abnormalities
The Plié 3 MPC Knee is appropriate for users who would benefit from the safety inherent in the
stability of a microprocessor controlled knee. These users should also have the ability or have
the potential to:
•negotiate obstacles in the community or workplace
•exert sufficient hip joint or pelvic voluntary muscle control
•ambulate with variable cadence
•descend stairs and ramps
1.2 Contraindications –Do not use Plié 3 MPC Knee on users with
insufficient hip joint or pelvic voluntary muscle control
insufficient cognitive ability to charge the batteries and care for the device
body weight exceeding 125 kg (260 lbs.) for moderate activity
body weight exceeding 100 kg (220 lbs.) for high activity
2. Symbols Used
CE MARK (European conformity)
Caution Symbol! Failure to adhere to this warning may result in user injury,
knee damage, or limit the device the function.
Type BF Applied Part used on device
Federal Communications Commission (USA conformity) used on device