Warning and Precautions
2102322-111 REV 1 Page 5
Warning and Precautions
WARNING
–
There is a risk of burns and injury to the patient. If an electrosurgery device is
used, disconnect the ECG leadwires from the patient.
•LEADWIRES –ECG Leadwires present a possible strangulation hazard. To avoid possible strangulation,
route all ECG Leadwires away from patient's throat.
•CONDUCTIVITY –Electric shock or device malfunction may occur if electrodes contact conductive
materials. Keep the conductive parts of lead electrodes and associated parts away from other
conductive parts, including earth. Also make sure that no contact to other conductive parts is possible
if the electrodes loosen during recording.
•GENERAL DANGER TO THE PATIENT –Instructions listed in this manual in no way supersede
established medical practices concerning patient care. Perform the establishedmedical practices
under all circumstances.
•EXPLOSION HAZARD—Do not use in the presence of a flammable anesthetic mixture withair or oxygen
or nitrous oxide.
•DEFIBRILLATION - Device is defibrillation protected when the original Norav Medical patient cable is
used. However, as a safety precaution when possible, remove theelectrodes before defibrillation.
•GENERAL DANGER TO THE PATIENT - The device is not designed for direct cardiac application.
•INFECTION RISK –Reuse of disposable parts that come into contact with patients pose a risk of
infecting patients. Do not reuse disposable parts that have had direct contact with the patient, such as
ECG electrodes.
•INTERPRETATION HAZARD - Computerized interpretation is only significant when used in conjunction
with clinical findings. A qualified physician must over read all computer-generated tracings.
•MAGNETIC AND ELECTRICAL INTERFERENCE - Magnetic and electrical fields are capable of interfering
with the proper performance of the device. For this reason, make sure that all external devices
operated in the vicinity of the device comply with the relevant EMC requirements. X-ray equipment or
MRI devices are possible sources of interference as theymay emit higher levels of electromagnetic
radiation. Ensure that there are no X-ray arrays, MRI or high-performance transformers are near the
device.
•OPERATOR - Medical technical equipment such as this system must only be used by qualified and
trained personnel. Operate the unit only at clinics and hospitals. Do not use at home.
•OPERATION WITH OTHER DEVICES
Other devices which are part of the system must meet the requirements of the Standard for
Information Technology Equipment (IEC/EN 60950-1) and the Standard for Electrical Medical
Devices (IEC/EN 60601-1).
The personal computer should be approved to the appropriate safety standard for non-
medical electrical equipment (IEC/EN 60950-1, or its national variants).
Accessory equipment connected to the analogue and digital interfaces must be certified
according to the respective IEC/EN standards (e.g. IEC/EN 60950-1 for data processing
equipment and IEC/EN 60601-1 for medical equipment). Furthermore, all configurations shall
comply with the valid version of the standard IEC/EN 60601-1. Therefore anybody, who
connects additional equipment to the signal input or output connector to configure a
medical system, must make sure that it complies with the standard.
When using GEH-ECG 1200 in combination with any other equipment, refer to aqualified
service technician for correct handling.
•MIXING UP RECORDINGS –To ensure that a recording is not assigned to the wrong patient DO NOT use
more than one identical marked RF ID pair of 1200W/1200WR at the same wireless environment.
!
Caution
•DAMAGE TO THE DEVICE THROUGH BATTERY LEAKAGE
–
Batteries may leak if left in an unused device
for prolonged periods. If you intend to store the device for longer than one week, remove the battery
from it.
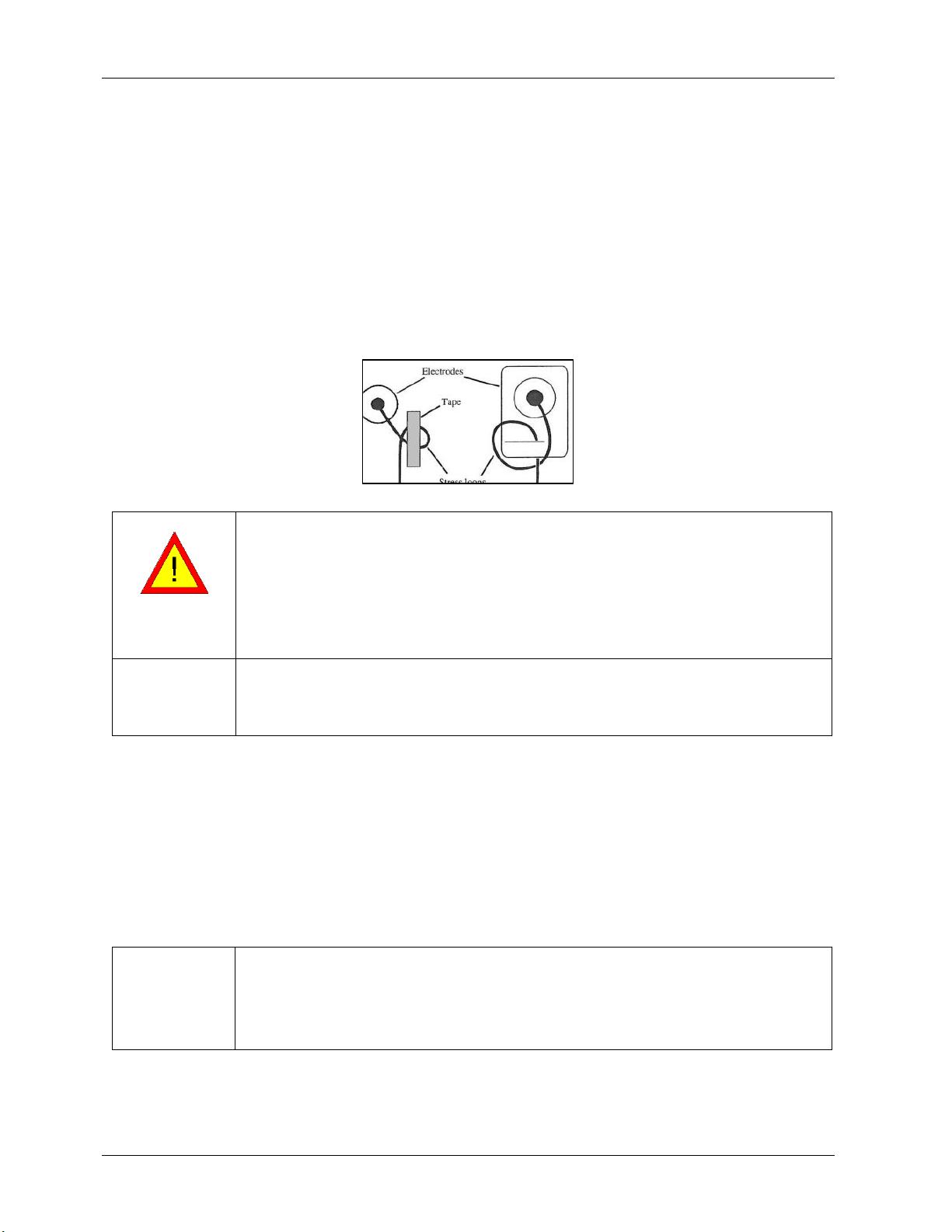
•LEADWIRE DAMAGE –Bending or wrapping the ECG Leadwire can damage it. When attaching and
affixing the ECG leadwire, make sure not to bend it excessively. Avoid coiling the ECG leadwire around
the device, as this can damage the leadwire.
•DAMAGE TO THE DEVICE –You may only open the battery compartment of the patient unit. Do not use
force when handling the recorder.
•SAFETY ONLY WITH APPROVED ACCESSORIES –Safe and reliable operation of the device is only possible
when using the supplied and approved accessories.
•DIFFICULTIES FINDING CAUSES FOR MALFUNCTIONS –To find and repair a malfunction, both device and
ECG leadwires are needed. Remember to include the ECG leadwire when returning the device for
service or repair. (Avoid wrapping the ECG leadwires around the device, as this can damage the
leadwires.) Always use the same ECG leadwires sets with a device. If an institution has several devices
and ECG leadwires sets, try to ensure that each device is matched with a specific ECG leadwires set. In
this way, leadwire or recorder failures can be isolated and eliminated faster. In the event of apparent
changes in the performance of the device, discontinue use immediately. Do not resume use until the
device is approved by the manufacturer or by a representative of the manufacturer.