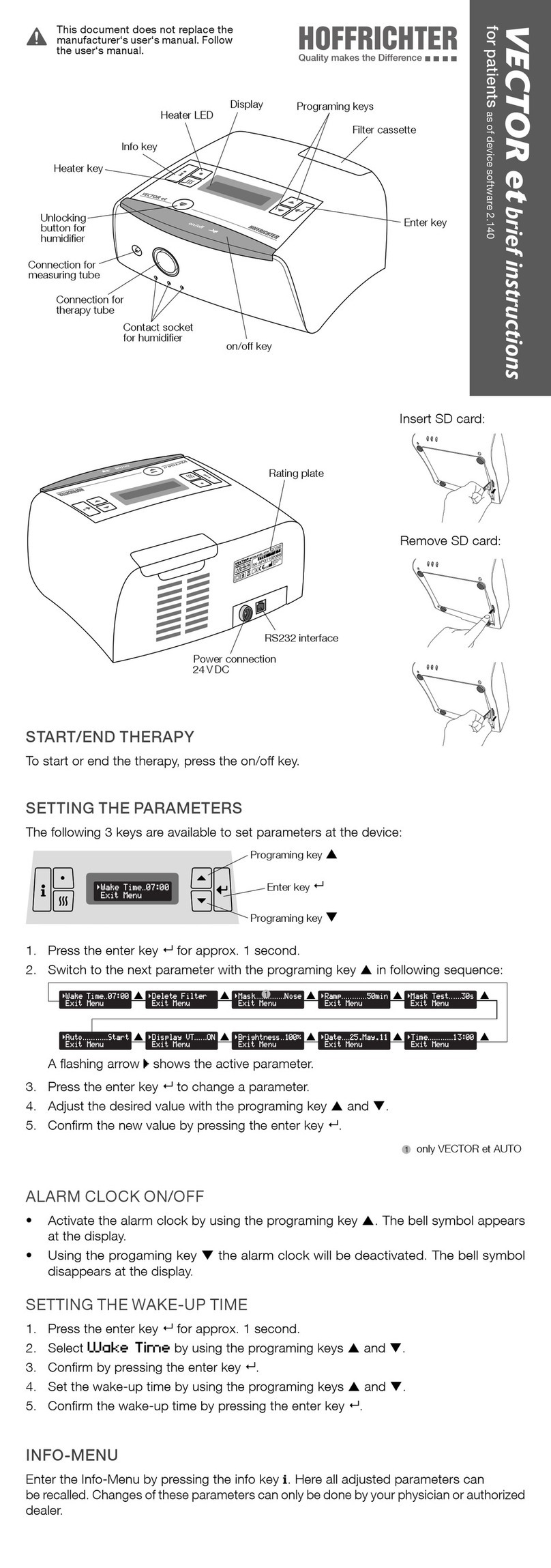
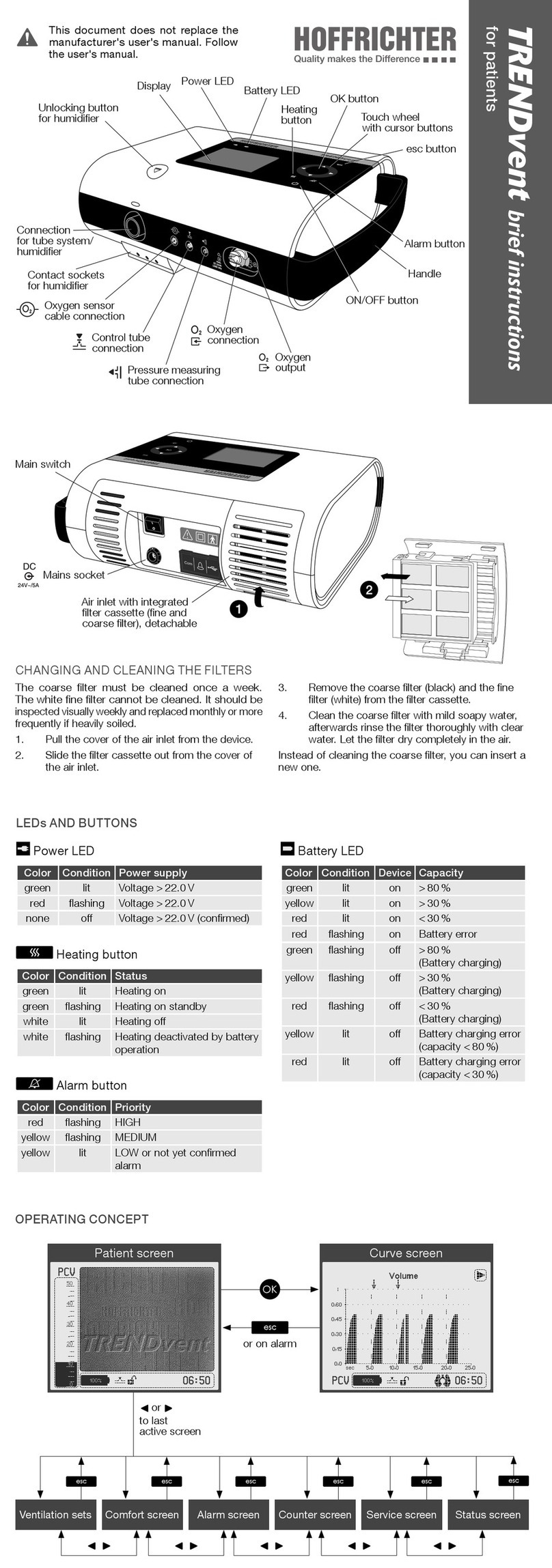
List of gures 9
Figure 37: PSV-S mode diagram...................................................................83
Figure 38: P-SIMV mode diagram .................................................................85
Figure 39: VCV mode diagram ......................................................................87
Figure 40: AVCV mode diagram ...................................................................89
Figure 41: V-SIMV mode diagram .................................................................91
Figure 42: CPAP mode diagram....................................................................92
Figure 43: Flow trigger (setting a percentage value) .......................................99
Figure 44: Inspiration trigger without SMART Function ................................100
Figure 45: Inspiration trigger using SMART Function ...................................101
Figure 46: User prole in the toolbar............................................................107
Figure 47: Basic screen layout ....................................................................112
Figure 48: Home screen .............................................................................115
Figure 49: Monitoring screen (data), factory setting .....................................118
Figure 50: Monitoring screen (graphs) .........................................................120
Figure 51: Monitoring screen (change the parameters) ................................121
Figure 52: Monitoring screen (freeze graphs) ...............................................122
Figure 53: Flow-Volume-Loop .....................................................................123
Figure 54: Volume-Pressure-Loop...............................................................123
Figure 55: Flow-Volume-Loop .....................................................................124
Figure 56: Monitoring screen (change the loop) ...........................................125
Figure 57: Parameter screen.......................................................................126
Figure 58: Parameter screen ......................................................................129
Figure 59: Alarm log screen ........................................................................132
Figure 60: System screen ...........................................................................134
Figure 61: Circuit conguration tightness check single line patient circuit .....139
Figure 62: Circuit conguration tightness check double line patient circuit ...139
Figure 63: Statistics screen (1 ventilation parameter) ...................................143
Figure 64: Statistics screen (2 ventilation parameter) ...................................144
Figure 65: "Measurements" night screen.....................................................145
Figure 66: "Light" night screen (with moon) .................................................145
Figure 67: "Dark" night screen (without moon).............................................145
Figure 68: Stop ventilation ..........................................................................147
Figure 69: Alarm displays in the toolbar.......................................................151
Figure 70: Alarm output in the toolbar .........................................................153
Figure 71: Alarm output in the textbox ........................................................153
Figure 72: Alarm box ..................................................................................154
Figure 73: Messages in the toolbar .............................................................160
Figure 74: Filter cassette structure ..............................................................165
Figure 75: Replacing valve membrane (expiration) .......................................172
Figure 76: Block diagram for the device ......................................................177
Figure 77: Pneumatic block diagram ...........................................................178