8 List of figures
List of figures
Figure 1: Rating plate (example)..................................................................... 14
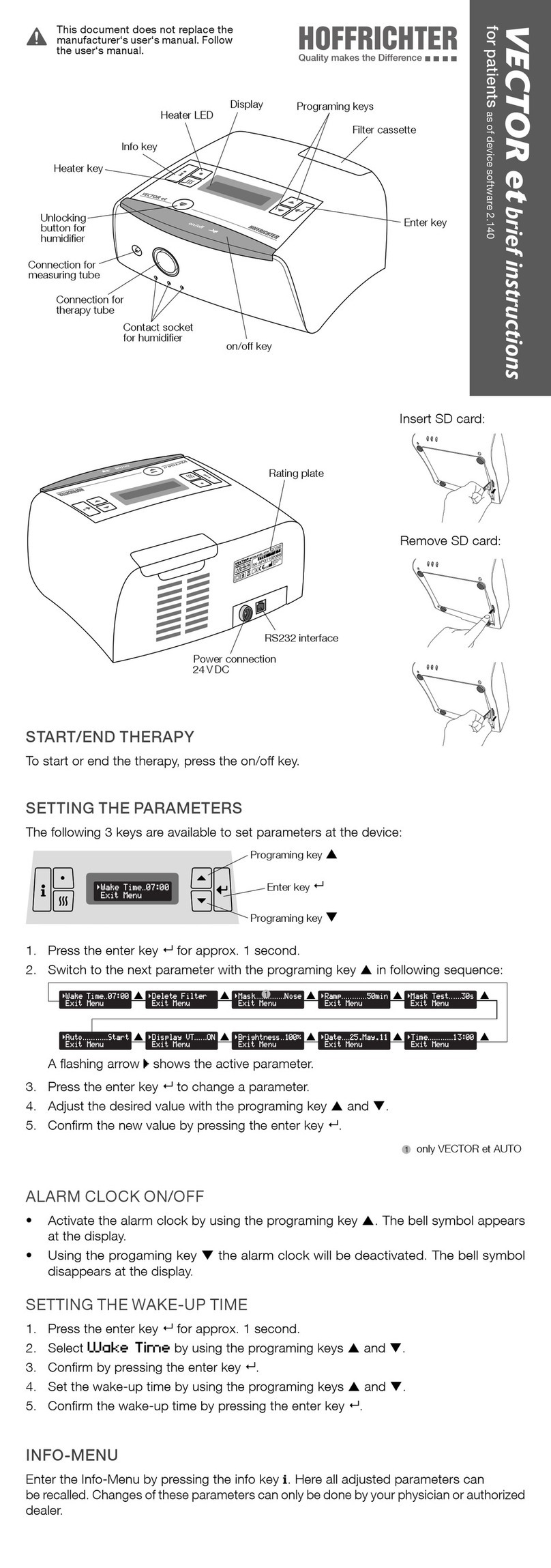
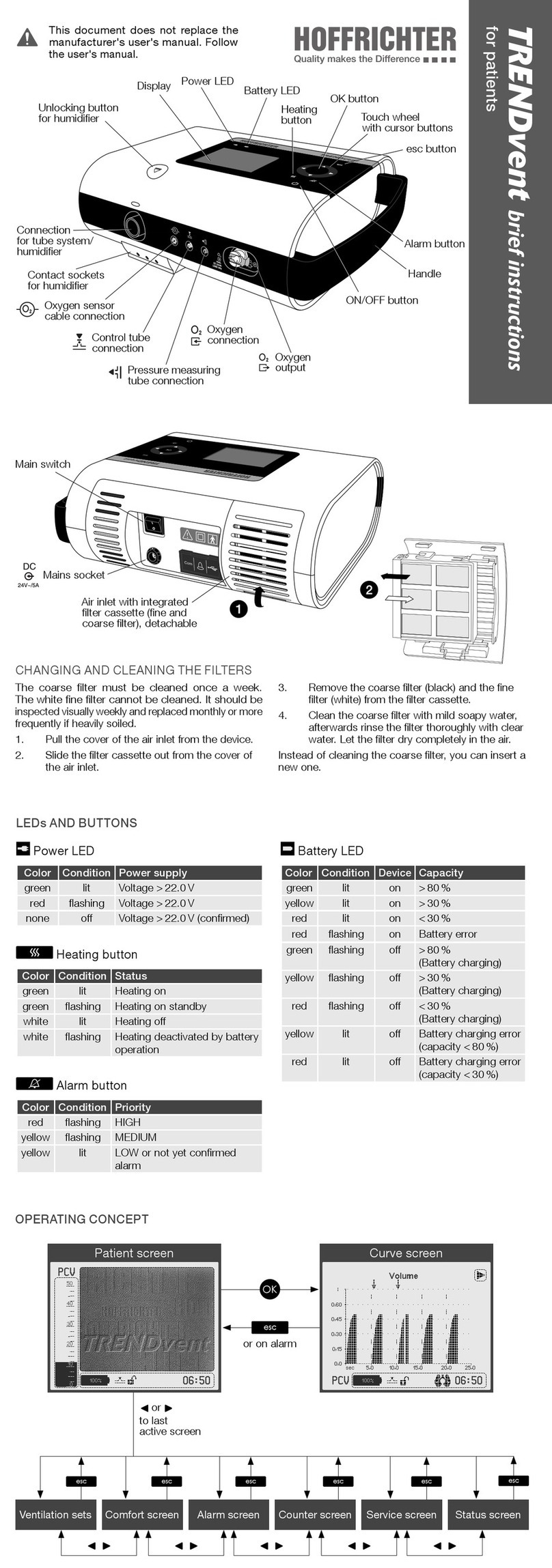
Figure 2: Top of device................................................................................... 30
Figure 3: Bottom of device............................................................................. 31
Figure 4: Rear of device ................................................................................. 32
Figure 5: Setting up the device....................................................................... 38
Figure 6: Mains connection via power supply unit.......................................... 39
Figure 7: Start screen ..................................................................................... 40
Figure 8: Connect Bluetooth adapter ............................................................. 41
Figure 9: System structure with CPAP mask .................................................... 42
Figure 10: Connecting AquaTREND III-NG........................................................ 43
Figure 11: Disconnecting AquaTREND III-NG from the device ........................... 46
Figure 12: Connecting the bacterial filter.......................................................... 49
Figure 13: Connecting a PC.............................................................................. 50
Figure 14: Inserting a USB stick ........................................................................ 51
Figure 15: nserting a USB stick......................................................................... 52
Figure 16: Supplying oxygen directly into the mask .......................................... 53
Figure 17: Supplying oxygen via an adapter ..................................................... 54
Figure 18: CPAP mask test and soft start .......................................................... 55
Figure 19: Example BILEVEL mask test and 20 min soft start ramp.................... 55
Figure 20: CPAP mode diagram........................................................................ 58
Figure 21: APAP mode diagram........................................................................ 59
Figure 22: Diagram of T mode without trigger function ................................... 60
Figure 23: Flow diagram of T mode without trigger function............................ 60
Figure 24: Diagram of T mode with trigger function......................................... 61
Figure 25: Flow diagram of T mode with trigger function................................. 61
Figure 26: Diagram of ST mode........................................................................ 62
Figure 27: Flow diagram of ST mode................................................................ 62
Figure 28: Diagram of S mode ......................................................................... 63
Figure 29: Diagram of A-S mode...................................................................... 64
Figure 30: Changing the user profile ................................................................ 67
Figure 31: PIN prompt when changing the user profile..................................... 67
Figure 32: Home screen................................................................................... 69
Figure 33: Standby screen................................................................................ 70
Figure 34: Monitoring screen, factory setting ................................................... 71
Figure 35: Comfort screen ............................................................................... 73
Figure 36: Therapy screen................................................................................ 73
Figure 37: System screen ................................................................................. 82
Figure 38: Reports screen in clinic mode: Ratings ............................................. 86
Figure 39: Reports screen in clinic mode: Indicators ......................................... 86
Figure 40: Reports screen in home mode: Ratings............................................ 87