2
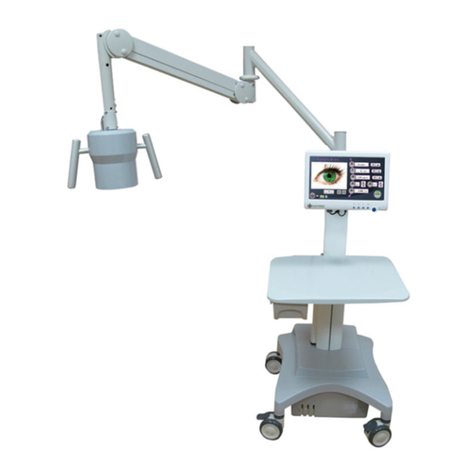
Service Manual for the LightLas 532 Medical Frequency
Doubled YAG Laser
Only authorized Service personnel should attempt to perform the procedures contained within
this Service Manual. The manufacturer can organise detailed Service Training for authorized
agents.
The manufacturer will under no account accept any responsibility for any conditions arising as
a result of unauthorized maintenance or adjustments to the LightLas 532 Medical Frequency
Doubled YAG Laser.
This Service Manual should be fully studied and understood before proceeding to operate or
service the equipment. For further details relating to the operation of the LightLas 532 Medical
Frequency Doubled YAG Laser the Service Manual should be referred to.
CAUTIONS - Use of controls or adjustments or performance of procedures other than
those specified herein may result in hazardous radiation exposure.
Any modification to the LightLas 532 Medical Laser will result in the necessity for it to be
reclassified.
CAUTIONS - US Law restricts this device to sale by or on the order of a physician.
This Service Manual contains confidential and proprietary information of the
manufacturer.
Manufactured by: LightMed Corporation, No.1-1, Ln. 1, Pao-An St.
Sec. 3,Shulin Dist., New Taipei City 23861, Taiwan
USA Address: 1030 Calle Cordillera, Suite 101, San Clemente, CA 92673
Tel No.: 949-218-9555 Fax No.: 949-218-9556
Copyright ©LightMed Corporation
EU Representative: Medical Device Safety Service GmbH
Schiffgraben 41, 30175 Hannover, Germany
Document Title: Service Manual for the LightLas 532 Medical Frequency Doubled YAG
Laser.
Document Number: DC2000-532
Document Revision History:
October 2001 - Draft prepared
March 2002 - Second version updated information
May 2002 - Third version with new Installation section, new drawings,
CE Mark information, Change to Revision 01
March 2003 - Rev.02 Modify drawings
February 2004 - Rev. 03 Safety Glasses description change; Delete Warranty
Statement
January 2005 - Rev. 04 Change EU Representative to ALF in ITALY