Multi-Wavelength Pattern Scanning System - LightLas 532 / 670 –Operators Manual Rev. No 01
Page 4 of 126
Table of Contents
Section 1 INTRODUCTION ……………………………………………………………..8
Section 2 SAFETY.………………………………………………………………………9
2.1 Product Classifications ….…………………………………………………………... 9
2.2 Warnings and Precautions …………………………………………………………. 10
2.3 Optical Hazards ……………………………………………………………………..12
2.3.1 Nominal Ocular Hazard Distance (NOHD)....................................................12
2.4 Electrical Hazards ……………………………………………………………………13
2.5 Safety Controls and Features ……………………………………………………….14
2.6 Product Labeling …………………………………………………………………….18
2.61 Console system............................................................................................... 18
2.62 Integrated Slit lamp LDU ..............................................................................20
2.63 Laser Indirect Ophthalmoscope LDU ........................................................... 22
Section 3 PRODUCT SPECIFICATIONS ……………………………...………………..23
3.1 Introduction ………………………………………………………………………… 23
3.1.1 Console Laser System ……………………………………………………...24
3.2.1 Laser Delivery Unit (LDU) ………………………………………………….25
3.3.1 Accessories …………………………………………………………………26
3.2 CSO SL980 Slit lamp Specifications …………………………………...…………...26
Section 4 PRINCIPLES OF OPERATION ….………………………………………..27
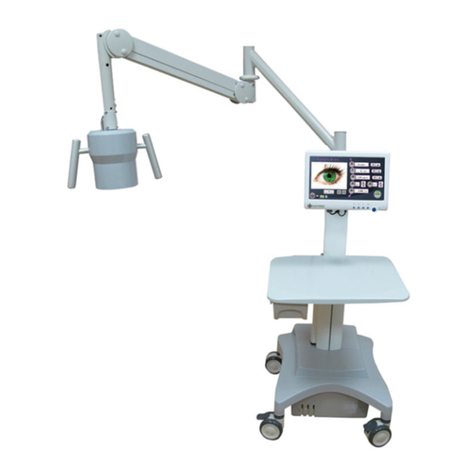
4.1 General Description ………………………………………………………………..27
4.2 Laser Console ……………………......……………………………………………..35
4.3 LCD Control Panel Display Screen……………………………………….................41
4.3.1 Console Control Screen…………………………………………………39
4.3.2 Pattern Control Screen ..............................................................................39
4.4. Remote Control Panel ............................................................................................. 53
4.5 Truscan Integrated into CSO model SL980………………………………57
A. Integrated CSO SL980 Controls Breakdown ……………………………59
4.6 Laser Indirect Ophthalmoscope (LIO).…………………………………...……60
Section 5 Installation………….…………………………………………………...63
5.1 Introduction ………………………………………………………………………...63
5.2 Unpacking and Receiving Inspection ……………………………………………...65
5.3 Tools and Equipment ………………………………………………………………66
5.4 Preparation …………………………………………………………...…………….67
5.4.1 System components …..…………..…………………………………… 67
A. System table top and motorized stand ………………………. ....……… 69
B. LDU system / LCD System / Footswitch / Accessories…..…… ... ………. 74
5.5 Final Verification / Checkout ……………………………………………………… 82
A. Slit lamp Integrity Rechecks ………………………………………………82