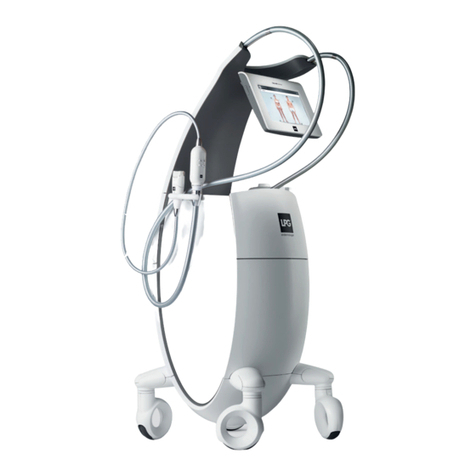
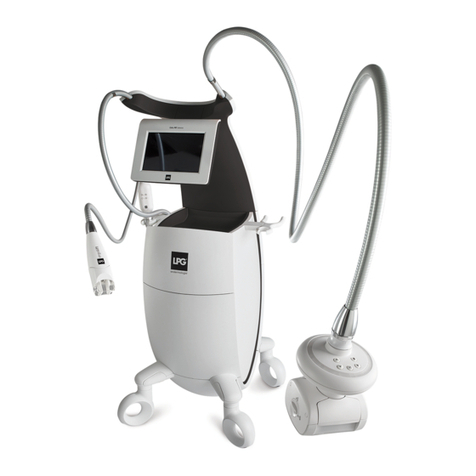
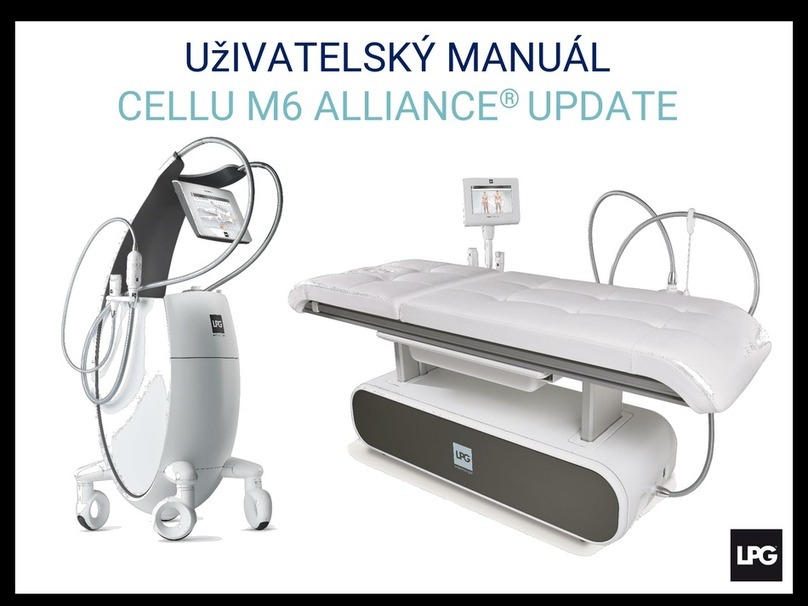
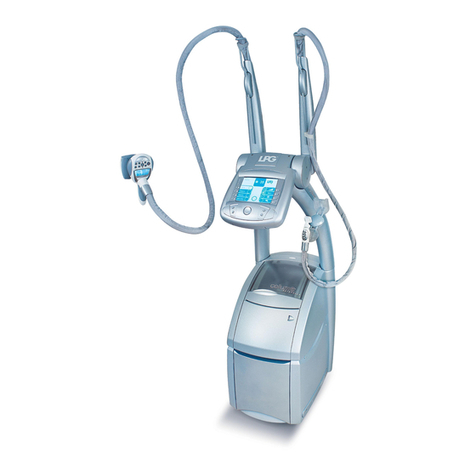
1010 11
Europe VII-H05VVF3G1,50-C19; Italy I/3/16-H05VVF3G1,50-C19; Switzerland 23G-H05VVF3G1.50-C19; UK
BS13/13-H05VVF3G1,50-C19; Japan 498GJ-VCTF3X2.00-C19; USA, Canada, Mexico N5/15-SJT3X14AWG-C19
(connect to Hospital grade receptacle in hospital environnement).
> IMPORTANT SAFETY INFORMATION
PRECAUTIONS FOR USE
3
PRECAUTIONS FOR USE
3
> PRECAUTIONS FOR USE
> IMPORTANT SAFETY INFORMATION
> WARNING
All safety precautions must be observed whilst using electrical equipment. Please read all
safety notices and precautions prior to use of the equipment.
• Never touch the client and the device’s
unprotected cables or connectors simultaneously.
• Never use the adapter as a treatment head or
allow it to come into direct contact with the skin.
• Only use treatment heads supplied with your unit
or recommended by LPG Systems.
• Do not use the treatment heads directly
on the skin. Wear the treatment suit provided by
LPG Systems, ENDERMOWEAR.
• LPG Systems will not be liable for any
inappropriate use of the equipment.
• Improper use of the device can cause tissue
damage or pain.
• The operator must be particularly attentive
to the sensations felt by the person undergoing
treatment.
• The operator must ensure that the parameters
(intensity, sequentiality, differential...) are always
adapted to the tissue being treated.
• Do not lean, rest, or sit on the unit.
• When crossing a threshold or step,
we recommend moving the unit carefully
by firmly holding the central arm monitor
stand to avoid the risk of tipping.
• Do not use the USB connection during treatment.
• Do not operate the unit in unsuitable
environmental conditions (see technical
specifications).
• The power plug is used as disconnect device.
The disconnection of the unit is disconnecting
the power plug.
• Please position your device so that the mains
unit is always accessible.
• Do not touch the client and the hose connectors
simultaneous
≥ATTENTION
• Always disconnect the equipment from
the electrical supply outlet after use
and before cleaning and maintenance.
• Check that the supply voltage of the unit
indicated on the data plate complies with
the mains voltage.
• The unit must be connected by the power
cord1 supplied to a grounded outlet
in accordance with current electrical
standards. Electrical adapters must
not be used with this equipment.
• Ensure that the unit is connected
to a system with a differential protection
against DC and AC.
DANGER - TO MINIMISE THE RISK OF ELECTRICAL SHOCK:
• TO MINIMISE THE RISK OF BURNS, FIRE,
ELECTRICAL SHOCK OR INJURY:
• The equipment must not be left unattended
whilst connected to the electrical supply.
• Disconnect the unit from the electrical
supply if it is not going to be used for
a long period.
• Special attention is required whilst using
the equipment with, or in the proximity
of children or disabled persons.
• Never use the unit for purposes other than
those recommended by LPG Systems. Only
use the treatment heads supplied with your
unit or recommended by LPG Systems.
• Never use the equipment if:
The electrical power cord or outlet is
damaged. The equipment does not function
correctly. The equipment is damaged or has
fallen or been dropped. The equipment has
been exposed to excessive humidity.
• Do not move the unit by pulling the electrical
power cord.
• Fully unwind the electrical power cord
and keep it away from warm surfaces.
• Never use the equipment if the ventilation
ports are obstructed. Ensure that
the ventilation ports are kept clear of dust
or other contaminants.
• Do not allow solid debris, liquid or other
foreign bodies to fall or be sucked into
the unit, as these could cause damage.
• Children shall not play with the device
• Cleaning and user maintenance shall not
be made by children without supervision
• Stop the device by using its main switch
when it is not use.
• Never use the equipment on a dusty,
uneven floor, or in a moist atmosphere.
• Never use the equipment in the presence
of aerosols or oxygen.
• Before disconnecting the unit from
the electrical supply, set all controls
to the ‘off’ position and unplug the unit.
The disconnection of the unit is
disconnecting the power outlet.
• It is prohibited to modify this equipment
without prior authorisation from
the manufacturer.
• It is prohibited to use components or spare
parts non recommended by LPG Systems.
• Return the device to LPG Systems Service
Center for examination and repair.
ATTENTION: KEEP THESE INSTRUCTIONS.
Your device should be used on healthy skin. It is important to read and respect the following
precautions and contraindications before using your device.