Magstim Rapid2 3576-23-09
The applied parts
of
this device, namely the stimulating coil,
i'v1EP
Pod and MEP Pod
patient leads, are electrically isolated from the otherparts
of
the equipment as required
by
the safety standards
ofBSEN60601-l.
2.2 INDICATIONS
The Rapid2 works by inducing electrical currents in tissue using a non-invasive stimulating coil at
frequencies
of
up to 100Hz. The stimulating coil is placed near the intended site
of
stimulation and
trigger pulses initiate
brief
magnetic pulses. The magnetic fields can pass through clothing, tissue and
bone to reach othenvise inaccessible areas. One feature
of
magnetic stimulation is that it
is
less likely to
stimulate pain fibres at the skin surface, reducing the discomfort when compared with conventional
electrical stimulation. Magstim Rapid2 magnetic stimulators combine stimulation frequencies from
lHz
to lO0Hz with a touch screen interface ·which controls every aspect
of
the stimulator's control and
operation.
INDICATIONS
FOR
USE. The Magstim Rapid2 magnetic nerve stimulator systems are intended
for the stimulation
of
peripheral nerves and the human cortex for
diagnostic ,md research purposes.
CONTRAINDICATIONS. Magstim Rapid2 systems and their accessories should not
be
used
on
or
in the vicinity
of
patients
or
users with cardiac demand pacemakers,
implanted defibrillators and/or implanted neurostimulators.
CAUTION. Check the Magstim Rapid2 System and its accessories for any signs
of
damage.
If
the Magstim Rapid2 System
or
any
of
its accessories are
damaged in any
way
they must not
be
used.
The MAGSTIM Company LTD
Federal law restricts this device to sale
by
or
on
the order
of
a
practitioner licensed
by
the law
of
the State in which he/she practices to
use or order the use
of
the device.
To avoid interference problems the Magstim Rapid2, and its
accessories, should not be used in the vicinity
of
any equipment that
does not comply with EMC Standard
EN
60601-1-2, including mobile
phones.
For
reasons
of
safety and reliability,
if
the system is set at
lOO'Y,,
pmver,
the nser must not exceed 250 stimuli per minnte,
or
4000 stimuli
per
hour
or
24,000 stimuli for every 24 hour period. This limitation is in
addition to any other limitation imposed
by
the dedicated controller,
or
heating effects on the stimulating coil and charger circuitry.
The entire Operating Manual should be studied prior to use and users
should be aware, in particular, that high voltages and currents, which
can prove lethal, are present
if
the covers are removed. The Magstim
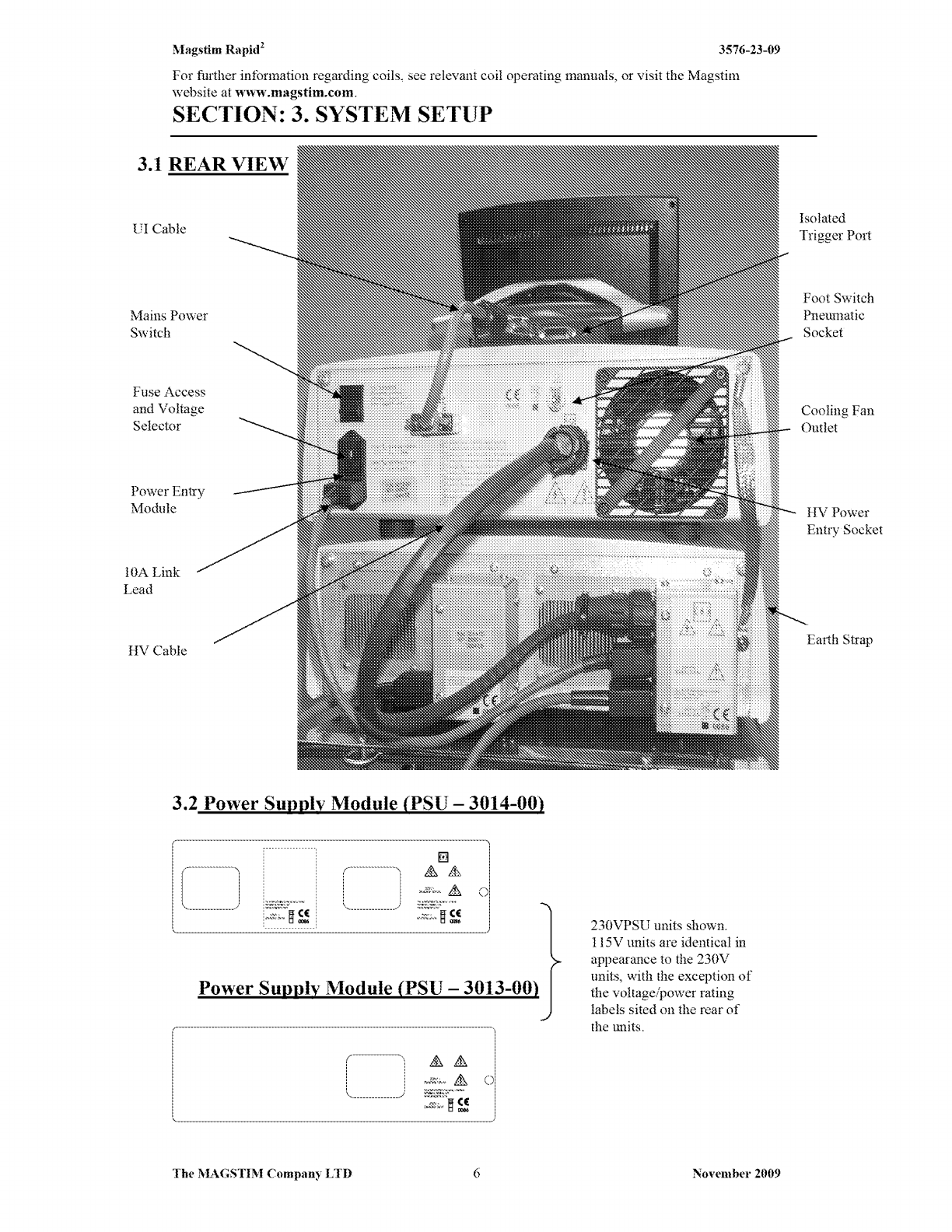
Rapid2 system's parts are detailed in Sections 3-6.
Each
of
the mains connections for the Rapid2 System must be made
via sepa1·ate, permanent, mains outlets.
On
no account should a
multi-way extension lead
be
used to connect more than one mains
connector to a single mains outlet.
The
Rapid2 System must be used only with the supplied mains
leads fitted with an integrnl filter, as they are requit-ed to maintain
the System's compliance with EN 60601-1-2 regarding Electro-
Magnetic emissions, as is the earth strap fitted between the
stimulator and the power supply unit (PSU).
The MEP
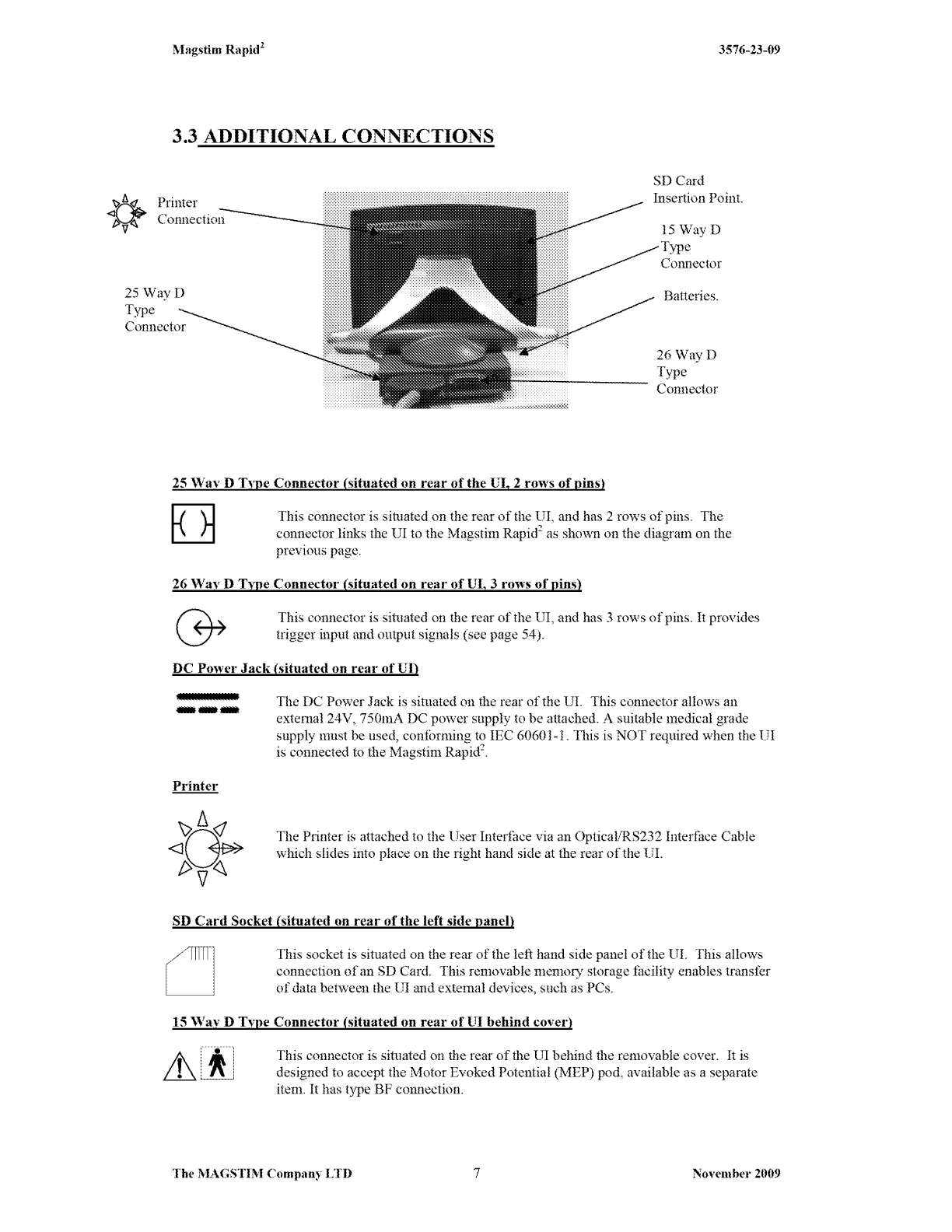
Pod
is designed to
be
fitted to the Magstim Rapid2
UI
only.
Do not attempt to connect it to a computer,
or
any other equipment,
v,rith
a similar connector. To do so could result in electric shock/ burns
to the patient at the site
of
electrode attachment. The MEP Pod
connector has a hole deliberately blocked to prevent incorrect
com1ection.
lOOHz
must
not
be used for cortical stimulation, it is for pel'iphernl
4 November 2009