GA 5752 4412 GB 17 3
Table of contents
Table of contents
1 Introduction ..............................................................................................................................................6
1.1 Foreword ....................................................................................................................................................6
1.2 How to use these operating instructions ....................................................................................................6
1.2.1 Abbreviations ............................................................................................................................... 6
1.2.2 Symbols ....................................................................................................................................... 6
1.2.2.1 Cross-references ....................................................................................................... 6
1.2.2.2 Actions and responses .............................................................................................. 6
1.2.3 Definitions .................................................................................................................................... 7
1.2.3.1 Design of safety notes ............................................................................................... 7
1.2.3.2 Structure of notes ...................................................................................................... 7
1.2.4 Explanation of pictograms, symbols and codes........................................................................... 7
1.3 Disposal......................................................................................................................................................9
1.3.1 Packing ........................................................................................................................................ 9
1.3.2 ATMOS products.......................................................................................................................... 9
1.3.3 Batteries and rechargeable batteries ........................................................................................... 9
1.3.4 Used electrical devices ................................................................................................................ 9
1.4 Overview ..................................................................................................................................................10
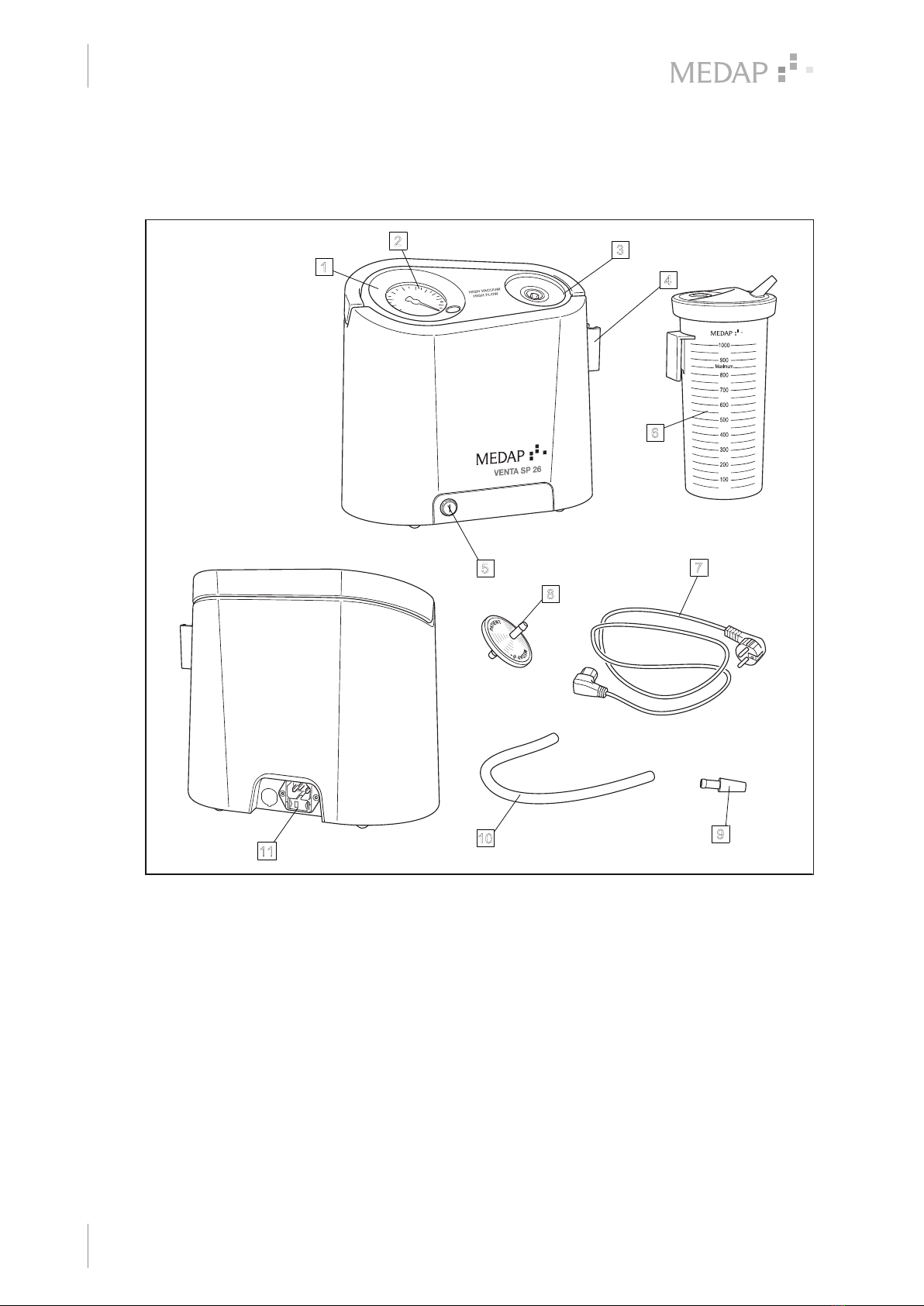
1.4.1 VENTA SP 26 N ......................................................................................................................... 10
1.4.2 VENTA SP 26 A.......................................................................................................................... 11
1.4.3 Trolley ........................................................................................................................................ 12
1.4.4 Aspirator holder, universal ......................................................................................................... 13
1.5 Basic requirements...................................................................................................................................13
1.5.1 Use in accordance with the intended purpose ........................................................................... 13
1.5.2 Applicable standards.................................................................................................................. 14
1.5.3 Intended purpose ....................................................................................................................... 14
1.6 Interface description.................................................................................................................................15
1.6.1 Hydrophobic bacterial and viral filter.......................................................................................... 15
1.6.2 Vacuum connection tube............................................................................................................ 15
1.6.3 Septic fluid jar including septic fluid jar cap ............................................................................... 16
1.6.4 Suction tube ............................................................................................................................... 16
1.6.5 Fingertip ..................................................................................................................................... 16
1.6.6 Utensil ........................................................................................................................................16
1.6.7 Connection of equipment mount ............................................................................................... 17
1.6.8 Application sets.......................................................................................................................... 17
1.6.9 Trolley ........................................................................................................................................ 17
1.6.10 Aspirator holder.......................................................................................................................... 17
1.7 Aspirator variants .....................................................................................................................................17