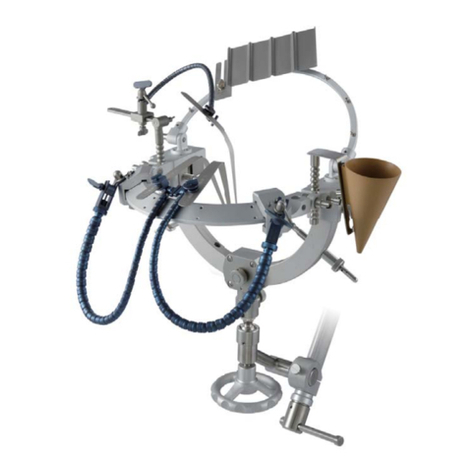
2/2
2. Service life of this product: 5 years
(Subject to following manufacturer's specified
maintenance, inspection and proper storage
requirements)
Maintenance/Inspection
1. Check prior to each use
Operational and functional checks
Conduct daily and pre-operation checks of this product to
make sure that it functions properly.
2. Check after each use
(1) Immediately wash with clean water.
(1)-1 If exposed to bleach or antiseptic solutions, wash
and rinse with clean water immediately and immerse
in neutral enzyme detergent to remove any bleach
or antiseptic solution, which may contain chlorine or
iodine and can damage the instrument. Manually
remove contaminated matter by hand or with an
ultrasonic-cleaner.
(1)-2 Further remove any remaining contamination with a
soft nylon brush.
(1)-3 Select a proper detergent for each decontamination
method and maintain appropriate density and
handling.
(1)-4 Use a soft towel, a soft nylon brush or a water jet for
cleaning.
(1)-5 To avoid damage, do not use a metal brush, coarse
polishing agents, or apply excessive force when
handling the device.
(1)-6 Only use distilled water or deionized water (reverse
osmosis) to wash this product.
(1)-7 Only use fully deionized water (reverse osmosis) for
the final rinse.
It is recommended to use a washer-disinfector for
this device.
Thermal Disinfection can be used by following the
manufacture's defined parameters.
Thermal Disinfection Band: 90-93°C/194.0-199.4°F,
5-10 minutes (A0 value: 3000-12000)
(reference EN ISO 15883-1)
(2) Fully dry this product immediately after washing it.
Do not leave it wet for a longer time than necessary as
residual water may damage the instrument.
(3) Only use distilled or deionized water
Use distilled or deionized water to wash this product.
Residual chlorine and organic matters in tap water may
cause staining and/or rust and may damage the
instrument.
(4) Use a water-based, anticorrosive lubricant
Lubricating oil is completely removed by washing. After
washing this product, apply a water-based, anticorrosive
lubricant prior to sterilization.
Do not use this product without applying anticorrosive
lubricant on its sliding part. [Galling could occur.]
3. Sterilization
Device must be sterilized by users in accordance with our
recommended sterilization procedures or the validated
sterilization conditions which validity is proven by medical
organizations in each country or region.
Our recommended sterilization parameters are as follows.
Sterilization
method
Pre-vacuum steam sterilization
(Autoclave sterilization)
Sterilization
conditions
Sterilization
temp.
Retention time
132°C / 269.6°F 4 min
134°C / 273.2°F 3 min
For the US market
FDA has not approved or cleared medical devices,
including sterilizers, for the intended use of reducing the
infectivity of TSE agents (i.e., prions).
For the markets outside the US
When the device is used on a patient with or suspected of
having CJD or vCJD, make sure to adhere to the most
recent and updated restrictions available in each country
and/or region for its reuse.
Maintenance and check by agents
MIZUHO Corporation will repair defective parts of this
product without charge for one year from the date of
delivery/installment except for cases of damage caused by a
third party’s repair, act of nature, improper use or intentional
damage. All other warranty terms and conditions are
subject to regulations of MIZUHO Corporation.
Packing
1 piece per pack
Matters related to warranty period
MIZUHO Corporation will repair defective parts of this
product without charge for one year from the date of
delivery/installment except for cases of damage caused by a
third party’s repair, act of nature, improper use or intentional
damage. All other warranty terms and conditions are subject
to regulations of MIZUHO Corporation.
Name and address of manufacturer
MIZUHO Corporation
3-30-13 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
http://www.mizuho.co.jp
Authorized Representative Europe
Emergo Europe B.V.
Prinsessegracht 20, 2514 AP, The Hague,
The Netherlands