1/2
MES-CK08-084-00EN-0
PREPARED: (2022-08-16) (Version 5)
Instruction for Use
Trade Name: Leg Strap
Warning
1. Please read these instructions carefully prior to using this product.
Product should be used according to these instructions, and pay
close attention to the safety of patients. Failure to follow
manufacturer's recommendations may cause harm or injury to the
patient.
2. For the US market
Do not reuse the device when it is used on a patient with or
suspected of having Creutzfeldt-Jakob Disease (CJD) or variant CJD
(vCJD).
3. For the market outside the US
When the device is used on a patient with or suspected of having CJD
or vCJD, make sure to adhere to the most recent and updated
restrictions available in each country and/or region for its reuse. Be
cautious of the possibility of secondary infection. Refer to
www.a-k-i.org or AAMI Standard ST79 for more information related
to cleaning and sterilization.
Contraindication/Prohibition
1. Use for intended purpose only
Use devices for their intended purposes only. Incorrect use could
cause this product to break.
2. Use with specified products only
Use this product only with products specified by Manufacturer. Other
products than those specified by Manufacturer could be incompatible
with this product due to differences in design and development
policies.
3. Prohibition of use of chemicals
Avoid exposing this product to chemicals. Doing so could damage
this product due to corrosion.
4. Prohibition of secondary processing of this product
Do not apply any secondary processing to this product. For example,
do not apply impacts or vibration markings to the surface of this
product. Doing so could break this product.
5. Handle with care
Handle this product with care, as it can be deformed or damaged.
Rough handling could significantly reduce the service life of devices
and appliances.
Symbol mark for labeling
MD :Medical Device
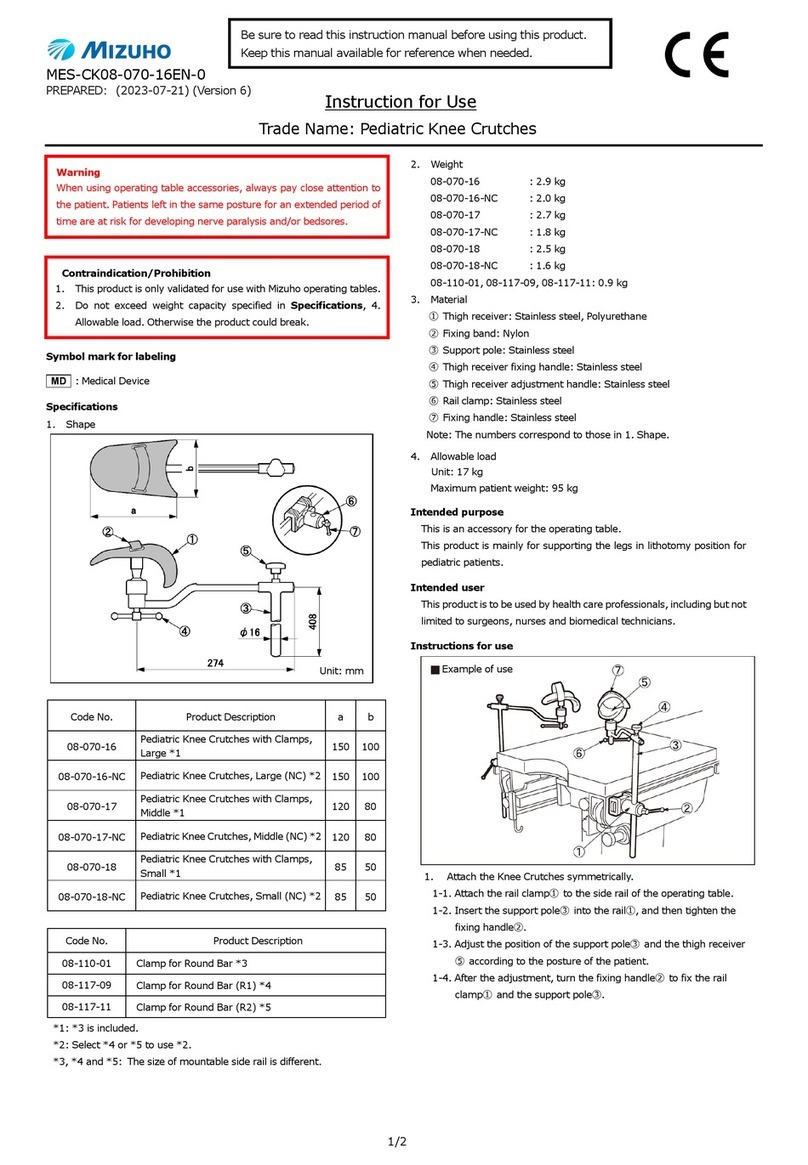
Specifications
Code No. Product Description
08-084-00 Leg Strap
Material: vinyl coated leather, Urethane, Nylon, Stainless steel
Intended purpose
This is an accessory to an operating table and is used to hold the legs of
a patient.
Intended user
This product is to be used by health care professionals, including but not
limited to surgeons, nurses and biomedical technicians.
Instructions for use
Be sure to inspect prior to using.
Buckle Pad Strap
1. Attach the pad to the patient in a manner appropriate for his/her
posture.
2. Wrap the strap around the operating table.
3. Run the strap through the buckle to secure the strap.
Warning/Caution
(1) Defect/Adverse event
Defect
・Deterioration, corrosion or pitting caused by use of chemicals
・Damage or breakage caused by the corrosion or pitting Adverse event
・Broken pieces of metal from the damaged instrument falling into the
patient.
(2) For hygiene, be sure to use sterile drapes on the areas on this
product where the patient comes into contact with it.
Storage/Life
1. Please store the device in normal ambient temperature areas. Do not
store in areas of high humidity where the temperature may
dramatically vary causing condensation. Do not store on or near
chemicals as the chemicals may cause damage to the device.
2. Service life of this product: This product is a consumable. Replace it
with a new product as soon as possible if deterioration of the
fastening force is observed.
Maintenance/Inspection
Check prior to each use
1. Operational and functional checks
Conduct daily and pre-operation checks of this product to make sure
that it functions properly.
2. After use, wipe with a clean, non-fluffy cloth moistened with alcohol.
If blood, etc., adheres, dispose of properly according to the laws and
guidelines of each country or region.
3. If you need to have this product repaired, consult your local dealer or
Mizuho.
Packing
1 piece per pack
Be sure to read this instruction manual before using this product.
Keep this manual available for reference when needed.