1/2
MES-CK07-932-11EN
PREPARED: (2023-10-03) (Version 1)
Instruction for Use
Trade Name: SUGITA AVM Microclip II CASE B
Warning
Please read these instructions carefully prior to using this product.
Product should be used according to these instructions, and pay close
attention to the safety of patients. Failure to follow manufacturer's
recommendations may cause harm or injury to the patient.
Contraindication/Prohibition
1. Use for intended purpose only
This product is intended to hold Sugita AVM Microclip II for
sterilization by a high-pressure steam. [Misuse may cause
damage.]
2. Prohibition of use of chemicals
Avoid exposing this product to chemicals. Doing so could damage
this product due to corrosion.
3. Prohibition of use of polishing powder and wire wool
When cleaning this product, do not attempt to polish its surfaces
with rough polishing powder or wire wool. This could cause
scratches on the surface of this product.
4. Prohibition of secondary processing of this product
Do not apply any secondary processing to this product, for
example, do not apply impacts or vibration markings to the surface
of this product. Doing so could break this product.
Specifications
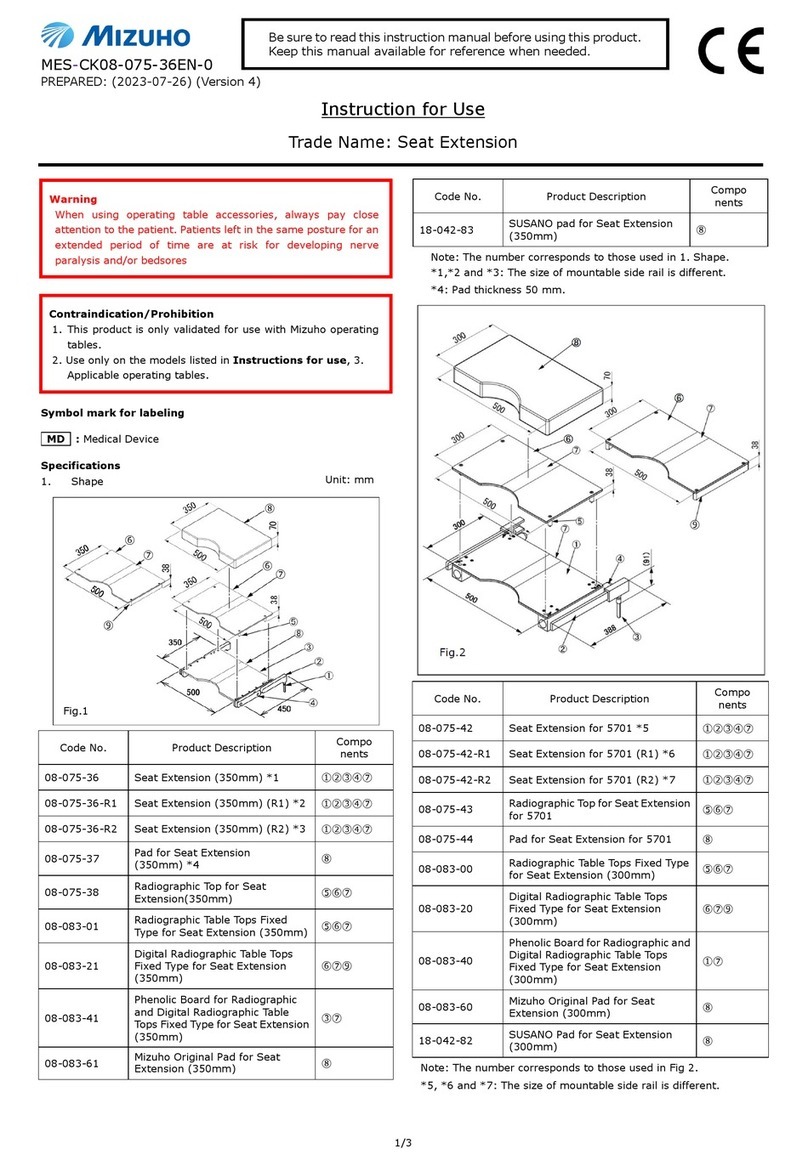
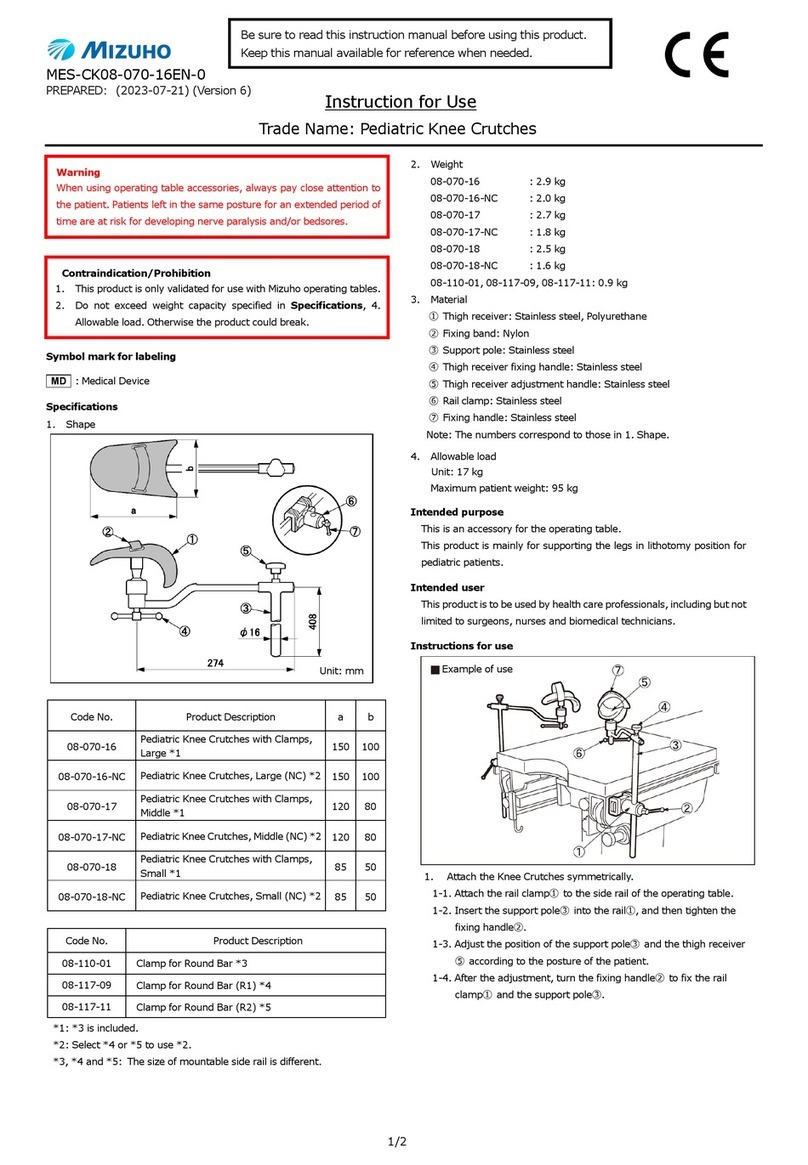
Code No. Product Description
07-932-11 SUGITA AVM Microclip II CASE B
Material: resin
Intended purpose
This product is a case that is used for washing the Sugita AVM Microclip
II and sterilizing it with high-pressure steam.
Intended user
This product is to be used by health care professionals, including but not
limited to surgeons, nurses and biomedical technicians.
Instructions for use
・Before using this product, inspect, wash, and sterilize in accordance
with these instructions.
・After setting the Sugita AVM Microclip II and the individual case in this
product, lock the lid with the grip.
・Sterilization of the Sugita AVM Microclip II can be performed with this
product by itself or by placing this product in "07-932-10: SUGITA
AVM Microclip II CASE A".
Warning/Caution
1. Important fundamental cautions
Device must be sterilized by users in accordance with our
recommended sterilization procedures or the validated sterilization
conditions for which validity has been proven by medical
organizations in each country or region.
2. Defect/Adverse event
Defect
・Deterioration caused by use of chemicals
Storage/Life
1. Please store the device in normal ambient temperature areas. Do not
store in areas of high humidity where the temperature may
dramatically vary causing condensation. Do not store on or near
chemicals as the chemicals may cause damage to the device.
2. Service life of this product: 5 years
(Subject to following manufacturer's specified maintenance,
inspection and proper storage requirements.)
Maintenance/Inspection
1. Check prior to each use
Operational and functional checks
Conduct daily and pre-operation checks of this product to make
sure that it functions properly.
2. Check after each use
(1) Immediately wash with clean water
(1)-1 If exposed to bleach or antiseptic solutions, immediately
wash:
Wash and rinse with clean water immediately and immerse
in neutral enzyme detergent to remove any bleach or
antiseptic solution, which may contain chlorine or iodine and
can damage the instrument. Manually remove
contaminated matter by hand or with an ultrasonic-cleaner.
(1)-2 Further remove any remaining contamination with a plastic
brush.
(1)-3 Select a proper detergent for each decontamination method
and maintain appropriate density and handling.
(1)-4 Use a soft towel, a plastic brush or a water jet for cleaning.
(1)-5 Avoid using metallic brushes or rough polishing agents,
applying excessive force, dropping or bumping the device,
etc.
(1)-6 Reverse osmosis water is recommended to wash this
product.
(1)-7 Only use reverse osmosis water for the final rinse.
(1)-8 It is recommended to use a washer-disinfector for this
device. Thermal Disinfection can be used by following the
manufacturer’s defined parameters.
Thermal Disinfection Band: 90-93°C/194.0-199.4°F, 5-10
minutes (A0 value: 3000-12000) (reference EN ISO
15883-1)
(2) Fully dry this product immediately after washing it
Do not leave it wet for a longer time than necessary as residual
water may damage the instrument.
(3) Use distilled water or reverse osmosis water
Use distilled water or reverse osmosis water to wash and sterilize
this product. Residual chlorine and organic matters in tap water
may cause stainings and/or rust and may damage the
instrument.
Be sure to read this instruction manual before using this product.
Keep this manual available for reference when needed.
Individual case
Grip
Lid