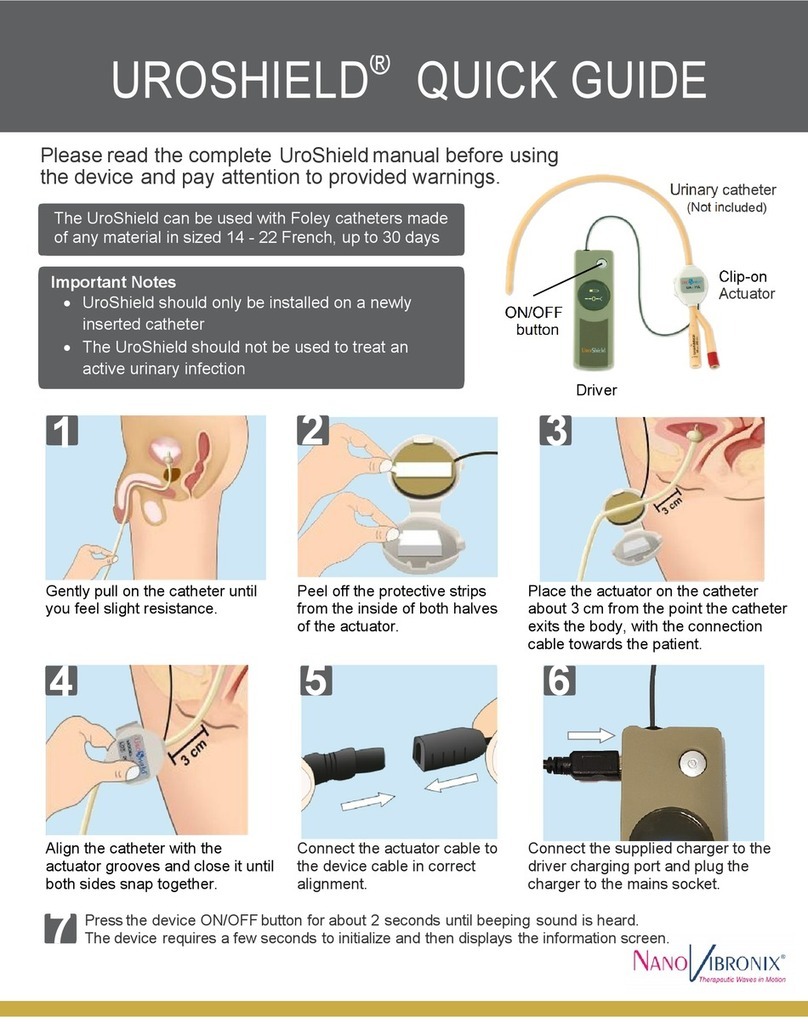
UroShield® User Manual
20-100-0022 Ver. 02 6
1 Introduction
Thank you for choosing the UroShield®. This manual contains general
instructions for operation, application, precautions and care. In order to obtain
maximum life and efficiency from the UroShield® and to assist in its proper
operation, please read and understand this manual thoroughly. The UroShield®
device is to be used only as directed in this manual.
1.1 Biofilm Formation and Urinary Tract Infections
Catheter-associated urinary tract infection (CAUTI) and other indwelling-device
associated infections are a major cause of morbidity and mortality in
hospitalized patients.
(1)
Urinary catheters readily acquire biofilms after
insertion; and the longer the catheter remains in place, the greater is the
tendency for the formation of biofilms, resulting in urinary tract infections.
The initial step in biofilm formation is the adhesion or attachment of planktonic
bacteria to the catheter surface. It is thought that the bacteria use touch
sensors to attach to a solid surface.
(2)
This occurs within a few hours after
urinary catheter placement. After attachment the bacteria begin to interlock, a
process known as docking. The bacteria then secrete an extra-cellular
polymeric matrix (ECM), which allows them to survive and proliferate. The
complex of the bacteria and ECM, now adherent to the catheter surface, is
known as biofilm. The established biofilm is highly resistant to antibiotics and
to the body's immune system.
The UroShield® is intended to prevent bacterial biofilm formation by
generating acoustic waves on the inner and outer surfaces of the catheter.
1
Pugach JL, DiTizio V, Mittelman MW et al; Antibiotic Hydrogel Coated Foley Catheters
for Prevention of Urinary Tract Infection in a Rabbit Model. The Journal of Urology,
Volume 162, 883-887, September 1999.
2
Princeton University News- Discovery of bacterial touch sensor could lead to Biofilm
treatments. http://www.princeton.edu/pr/news/02/q1/0205-touchsensor.htm.