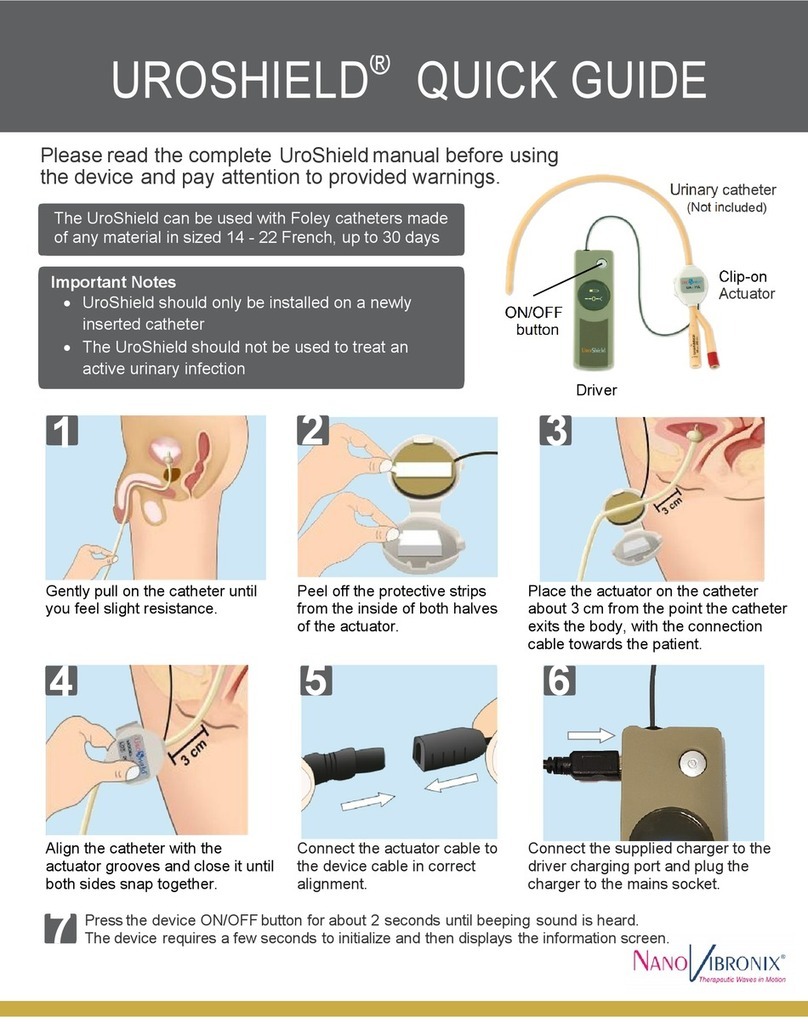
PainShield ®
Attention – this guide provides the basic principals of operation for the PainShield ®device. It is not intended to replace the complete
user manual. Please read the complete manual (PSUM003) before using the PainShield and make sure that you understand all
aspects of PainShield operation.
The PainShield MD is a microprocessor controlled therapeutic ultrasound device that produces low frequency , low intensity ultrasonic waves for the relief of
pain , muscle spasm and improve ment of localized blood circulation. The ultrasonic waves are generated by a unique transducer incorporated into a
treatment patch. There are two types of the patches: Reusable treatment patch – A and Reusable facial treatment patch – B . The t reatment patch should be
placed on the skin at the desired treatment area or adjacent to it .
For maximal effectiveness – ensure that the ultrasonic transducer is in full contact with the skin .
Pain Shield MD
model FD -14A specifications
Contraindications
Do not use in patients with cancer and bone metastases
Do not apply over bone growth centers until bone growth is complete
Do not apply directly on the eye
Do not apply directly over ischemic tissues in individuals with vascular disease
Pregnant women should be treated only wit h physician consent.
Precautions
In children, it is preferable to avoid usage over the epiphyseal growth plate area.
Following a laminectomy when major tissue removal has occurred
Over anesthetic areas
When hemorrhagic diathesis is present
General warnin gs and precautions
Treating of children should be done under adult supervision.
Following treatment, some redness might occur in the treated area. The redness should resolve naturally within a few hours.
Do not place the treatment patch directly on open w ound.
The safety and effectiveness of PainShield has not been determined in patients who are being treated by, or who have received , other medical
devices including but not limited to pacemakers, electrical stimulators, radiofrequency generators, surgical meshes
or other surgical implants.
PSIFU009 Ver. 03
USA
– NanoVibronix Inc., 105 Maxess Road, Suite S124 Melville, NY 11747 , Tel: +631 -574 -4410
Europe
- NanoVibronix Ltd. 9 Derech Hashalom S t., Nesher 36603, Israel , Tel: +9 72 4 820 -0581
www.nanovibronix.com info@nanovibronix.com